Abstract
The authors report on the design and synthesis of new semiconducting materials, based on a triphenylene core. In comparison with all previous reported triphenylene derivatives, these molecules have lower bandgap energies. 2,3,6,7,10,11-Hexakis(4-((E)-2-(5'-hexyl-2,2'-bithiophen-5-yl)vinyl)phenyl) triphenylene and 2,3,6,7,10,11-Hexakis(4-((E)-2-(5'-dodecyl-2,2'-bithiophen-5-yl)vinyl)phenyl)triphenylene have good solubility and are applicable to soluble processing for organic field effect transistor (OFET) and organic solar cell (OSC) fabrications. As far as we know, there are few reports on the application of π-extended triphenylene systems to OFET. The other molecule, 2,3,6,7,10,11-Hexakis((4-(5-dodecylthiophen-2-yl)phenyl)ethynyl)triphenylene, is a new discotic liquid crystalline (LC) semiconducting material. This molecule is readily soluble in common organic solvents to exhibit self-organizing long fiber structures with supramolecularly ordered columnar stacks that lie parallel to the substrate. The semiconducting properties of these molecules were quite promising for further designs of π-extended triphenylene derivatives.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
There has been growing interest in π-conjugated systems over the last several years due to their unique semiconducting and electro-optical properties and because of their potential applications in different electronic devices, such as organic field-effect transistor devices (OFETs) [1, 2] and organic solar cells (OSC) [3].
Compared with the linear organic conjugated oligomers and polymers, multi-branched molecules are much more advantageous. The synthesis of π-conjugated multi-branched molecules raises the possibility of creating thiophene derivatives that are fully tethered to the aromatic core, which can be synthesized by a convergent method. Furthermore, the solubility problem in linear conjugated oligothiophenes is mostly overcome under a unique molecular architecture [4–8].
Columnar discotics are an interesting class of materials that can be potentially used as organic semiconductors in electronic devices. The corresponding mesogens consist of an aromatic or fused aromatic core, which can be chemically modified by peripheral substitution and self-assembly into one-dimensional columnar superstructures that then arrange in a two-dimensional lattice. The overlapping of the π-orbitals of adjacent molecules within the columns ensures that one-dimensional intra-columnar charge-carrier transport occurs. While charge carrier mobilities that are more than sufficient for thin film transistor applications have been reported, utilization of the discotic liquid crystalline molecule will be realized only when the pathways for transport can be uniaxially aligned parallel to the surfaces in thin film geometries that have length scales of at least 100 nm [9, 10].
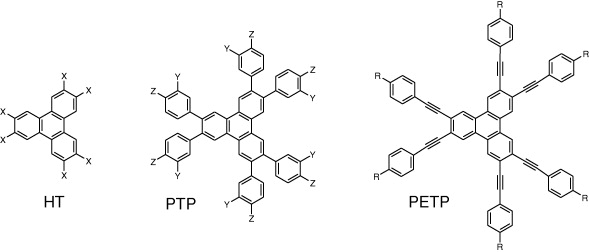
Triphenylenes and their derivatives have been intensively synthesized and investigated in recent decades. Such molecules have attracted attention mainly because of their unique tendency to aggregate into geometrically confined one-dimensional columnar arrays that can display liquid crystalline properties [11]. Hexaalkoxytriphenylenes, hexaalkylthio-triphenylenes, hexaphenyltriphenylenes and hexaphenyl-ethynyl-triphenylenes have been synthesized as its derivatives [12–20] (figure 1). However, these derivatives show high bandgap energies that would weaken their potential in electronic applications.
Figure 1 Molecular structures of reported triphenylene molecules. (X=OC n H 2n+1 or SC n H 2n+1); Y, Z=OC n H 2n+1, C n H 2n+1 or H; R=C n H 2n+1) [12–20].
In this work, the authors report the design and synthesis of new semiconducting materials, based on a triphenylene core. In comparison with the previous reported triphenylene derivatives, these molecules showed relatively lower bandgap energies with good crystallinity. The bandgap energies were so low that we can use these materials as a conducting layer in molecular electronic devices, although the fabrication conditions of the devices were not yet fully optimized.
2. Experimental
2.1. Synthesis of π-extended triphenylenes
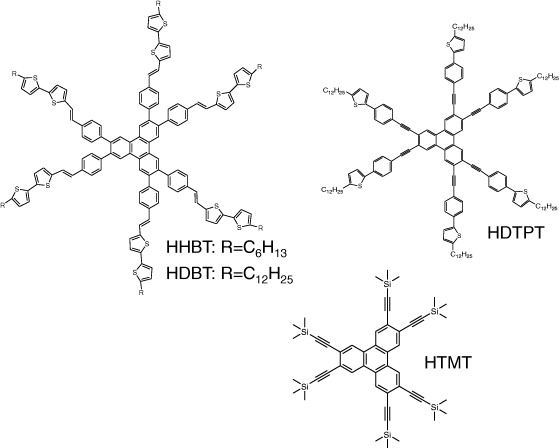
We carried out the facile and high-yield synthesis of the new p-type thiophene-based π-extended triphenylenes. 2,3,6,7,10,11-hexabromotriphenylene was prepared according to the literature procedure [21]. In figure 2, 2,3,6,7,10,11-Hexakis(4-((E)-2-(5'-hexyl-2,2'-bithiophen-5-yl)vinyl)phenyl) triphenylene (HHBT) and 2,3,6,7,10,11-hexakis(4-((E)-2-(5'-dodecyl-2,2'-bithiophen-5-yl)vinyl)phenyl) triphenylene (HDBT) were prepared by Horner–Emmons coupling reaction of dodecaethyl (4,4',4'',4''',4'''',4'''''-(-(triphenylene-2,3,6,7,10,11-hexayl) hexakis (4,1-phenylene))hexakis(methylene) hexaphosphonate with 5'-hexyl-2,2'-bithiophene-5-carbaldehyde and 5'-dodecyl-2,2'-bithiophene-5-carbaldehyde, respectively.
Figure 2 Molecular structures of synthetic materials HHBT, HDBT, HDTPT and HTMT.
2,3,6,7,10,11-hexakis((4-(5-dodecylthiophen-2-yl)phenyl)ethynyl)triphenylene (HDTPT) and 2,3,6,7,10,11-hexakis((trimethylsilyl)ethynyl)triphenylene (HTMT) were prepared by Sonogashira coupling reaction of 2,3,6,7,10,11-hexabromotriphenylene with 2-dodecyl-5-(4-ethynylphenyl)thiophene and ethynyltrimethylsilane, respectively.
2.2. Characterization and measurements
1 H NMR spectra were recorded on a Varian Mercury NMR 400 Hz spectrometer using deuterated chloroform purchased from Cambridge Isotope Laboratories Inc. 13 C NMR spectra were recorded using a Varian Inova-500 spectrometer. Elemental analysis was performed by using an EA1112 (Thermo Electron Corp.) elemental analyzer. Mass analysis was performed on a JMS-AX505WA (JEOL) mass spectrometer. MALDI-TOF analysis was performed on a Voyager-DE STR MADI-TOF (matrix; DHB) mass spectrometer. Thermal properties were studied under a nitrogen atmosphere on a Mettler DSC 821e and Mettler TGA 50.
The redox properties of the molecules were examined by using cyclic voltammetry (Model: EA161 eDAQ). Thin films were coated on a platinum plate using chloroform as a solvent. The electrolyte solution employed was 0.10 M tetrabutylammonium hexafluorophosphate (Bu 4 NPF 6) in a freshly dried DCM. The Ag/AgCl and Pt wire (0.5 mm in diameter) electrodes were utilized as reference and counter electrodes, respectively. The scan rate was 50 mV s −1. Fourier transformed-Infrared (FT-IR) spectra were recorded using a Nicolet 380 infrared spectrophotometer.
Absorption spectra of chloroform solutions were obtained using a UV-Vis spectrometer (HP 8453, PDA type) in the wavelength range of 190–1100 nm. PL spectra were recorded with a Hitachi's F-7000 FL Spectrophotometer.
Atomic force microscopy (AFM) operating in tapping mode with a silicon cantilever was used to characterize the surface morphologies of the samples. The film samples were fabricated by spin-coating (1500 rpm) on silicon wafer followed by drying under vacuum (solvent: chloroform, solution concentration: 10 mg ml −1).
The field effect transistors consisted of heavily doped 〈100〉 silicon that we used as the gate electrode, with 300 nm thermally grown SiO 2 used as gate insulator. The source and drain electrodes (120 nm Au) were deposited by thermal evaporation through a shadow mask. We washed the wafers with acetone and isopropanol, followed by ultraviolet-ozone exposure to clean the wafers. The silicon oxide was treated with octyltrichlorosilane (OTS) by immersing freshly cleaned wafers in an 8 mmol l −1 solution of OTS in anhydrous toluene for 30 min, followed by sonication in toluene and isopropanol, consecutively. The film devices were fabricated by directly spin-coating a 1% solution of semiconductor materials onto the dielectric substrate at 1600 rpm.
3. Results and discussion
3.1. Identity and structure of the synthetic materials
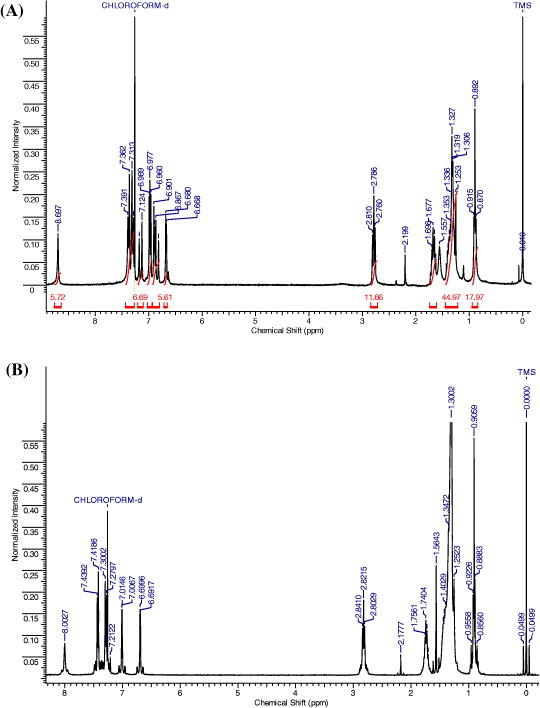
The identity and purity of the synthetic materials were confirmed by 1 H NMR (figure 3), MALDI-TOF mass spectrometry and elemental analysis. They were found to have good self-film-forming properties and well-soluble in various organic solvents such as chloroform, dichloromethane, ethyl acetate, chlorobenzene and tetrahydrofuran.
Figure 3 1 H NMR spectra of HHBT (A) and HDTPT (B).
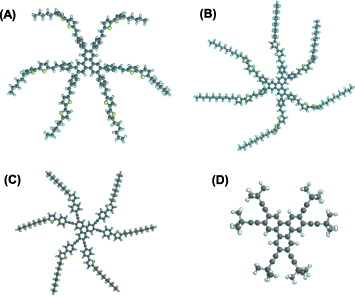
Furthermore, computer calculations using the density functional theory (DFT) model incorporated into the Spartan program ('06) were performed to determine the minimum energy conformations for four molecules HHBT, HDBT, HDTPT and HTMT [22]. The optimized geometrical structures of four molecules are illustrated in figure 4. The shapes of two molecules HHBT and HDBT are expected to be quite similar. HHBT has a diameter of 45 Å while HDBT has a diameter of 57 Å, which indicates that the core part seems to be quite planar even though six benzene rings are twisted with the triphenylene core. By contrast, the geometry of HDTPT and HTMT showed that the π-extended core had an absolutely planar structure, which is a prerequisite for the uniaxial orientation of columnar discotic liquid crystals.
Figure 4 Optimized geometries of four molecules: (A) HHBT, (B) HDBT, (C) HDTPT and (D) HTMT.
The absorption spectra of HHBT and HDBT showed an absorption maximum at 401 nm in solution state and at 406 nm in film state, respectively. The absorption of HDTPT in a dilute chloroform solution showed an absorption maximum at 382 nm. A drastic spectral change was observed in the film state of this molecule, which is attributed to a high degree of intermolecular interaction. In comparison with the previous reported triphenylenes derivatives, these molecules showed red-shifted absorption spectral behavior arising from lower bandgap energies in the solid state. The absorption and photoluminescence (PL) maxima, , energy levels of these molecules are summarized in table 1. With ΔE g s of 2.5–2.6 eV at film state, these molecules can be used for OFET and OSC fabrication.
Table 1. Optical properties and cyclic voltammetry analysis data of HHBT, HDBT and HDTPT.
| HHBT | 401 | 454 | 406 | 474 | 464 | 520 | −5.22 | −2.49 | 2.73 | |||
| HDBT | 401 | 453 | 406 | 472 | 464 | 515 | −5.24 | −2.51 | 2.73 | |||
| HDTPT | 382 | 430 | 394; 420 | 485 | 439 | 500 | −5.21 | −2.33 | 2.88 |
The thermal properties of HHBT, HDBT and HDTPT were characterized by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). HHBT displayed a distinct melting temperature at 215 °C, but it does not show clear crystalline transition in the cooling cycle. By contrast, HDBT exhibited two endothermic peaks at 139 and 162 °C and an isotropic-crystalline transition at 138 °C. HDTPT displayed an endothermic peak at 67 °C and a clear crystalline transition at 22 °C in the cooling cycle. TGA measurements at a heating rate of 10 °C min −1 under nitrogen revealed that HHBT, HDBT and HDTPT had good thermal stabilities. They showed the onset decomposition temperature in the range of 360–390 °C. The melting temperature, crystallization temperature and decomposition temperature of HHBT, HDBT and HDTPT are summarized in table 2.
Table 2. Physical properties of the three molecules HHBT, HDBT and HDTPT and OFET device performance.
| HHBT | 392 | 215 | – | 1.4 (±0.2) | 1000 |
| HDBT | 385 | 162 | 138 | 0.28 (±0.02) | 1000 |
| HDTPT | 360 | 67 | 22 | – | – |
3.2. Optical and atomic force microscopic study of π-extended triphenylenes
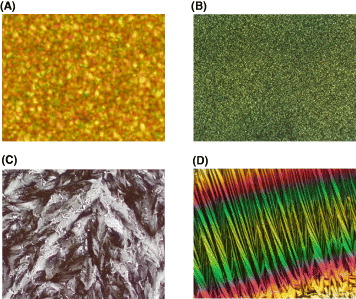
By using a hot-stage optical microscope (Mettler Co.), the crystalline shape and LC properties of these molecules were observed under crossed polarizers. Depending on the temperature for annealing, we could observe different LC textures. Figure 5 shows the crystalline structures of four molecules after inducing crystallization. Under the same magnification, the crystallites of HHBT are significantly bigger than those of HDBT. Figure 5(D) also shows highly ordered LC columnar phase of HDTPT (×400 times). The molecule can be processed from solution to form self-organizing long fibers with supramolecularly ordered columnar stacks that lie parallel to the substrate and can be oriented uniaxially onto normal glass slides. The intermolecular coupling of π-electrons on adjacent molecules along the columnar stack in discotic liquid crystals has been recognized as a promising structure that can allow easy charge movement along the stack. The fabrication of a field effect transistor using highly ordered HDTPT is now in progress.
Figure 5 Optical micrographs of four molecules: (A) solid crystal of HHBT, (B) solid crystal of HDBT, (C) solid crystal of HTMT and (D) liquid crystal of HDTPT.
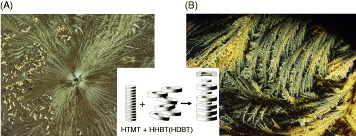
Figure 6 shows the crystalline structures of mixtures of HHBT or HDBT into columns of HTMT after recrystallization. This is complementary polytopic interaction (CPI) columnar mesophases, in which the columns are built up of alternating HTMT and HHBT or HDBT molecules. The crystallinity of HHBT or HDBT is substantially enhanced by adding one mole of HTMT. The modeling was shown in the inset of figure 6. The columns are highly ordered, and consequently charge-carrier mobilities along the column are high.
Figure 6 Optical micrographs of composite of (A) HHBT, (B) HDBT with HTMT (1/1 molar ratio). The inset figure is a schematic representation of the intercalation of HHBT or HDBT into columns of HTMT. The HTMT molecules fit into the 'hollow' in the face of the HHBT and HDBT.
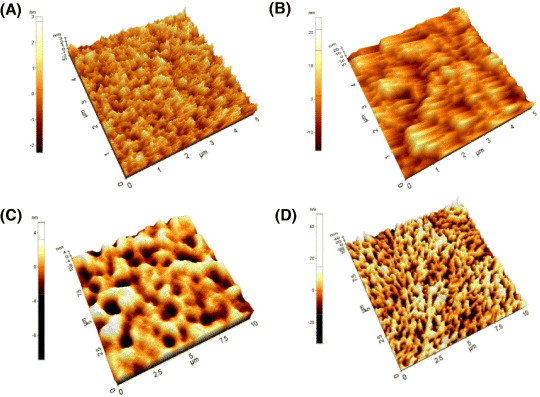
Further study of film morphology was obtained by AFM (figure 7). The solutions of the molecules were spin-coated on a silicon wafer and annealed at different temperatures. HHBT showed the compact structure on the surface with the smaller crystallites (figure 7(A)). A high coverage of the substrate was achieved simply by spin coating. This is because the HHBT bearing hexyl group has an effective interdigitation yielding lamella ordering and rigid conformation. By contrast, the HDBT bearing dodecyl group has a worse crystalline packing, which might be due to the hairpin conformation of the dodecyl group at the periphery (figure 7(B)). It can be thought that the highly packed crystalline molecules on the dielectric layer can help the carrier transport with good connection behavior.
Figure 7 AFM topographic images of three molecules: (A) HHBT, (B) HDBT, (C) HDTPT at RT and (D) HDTPT at 140 °C.
Figures 7(C) and (D) show the surface coverage of HDTPT at room temperature (RT) and after annealing at 140 °C. Before annealing, the HDTPT bearing dodecyl group has worse crystalline packing. However, after annealing, it showed the compact structure on the surface with highly ordered and rigid conformation. It is expected that, after annealing at melting state and cooling to RT, the liquid crystals were frozen with supramolecularly ordered columnar stacks.
3.3. Properties of OFET made of multi-branched conjugated molecules
Bottom-contact OFET devices were fabricated using gold source and drain electrodes, which were thermally evaporated using the conventional method. The devices have 5 μm channel length and 1500 μm channel width. A thin film (∼40 nm) of the semiconductor was deposited by spin coating a 2 wt% solution of these molecules in monochlorobenzene. In order to achieve optimal performance, the OFET devices made of HHBT and HDBT were further annealed at 170 and 140 °C, respectively, for 30 min. FET properties were measured at room temperature. The field-effect mobilities, μ, can be calculated from the amplification characteristics, by using the classical equations describing field-effect transistors.
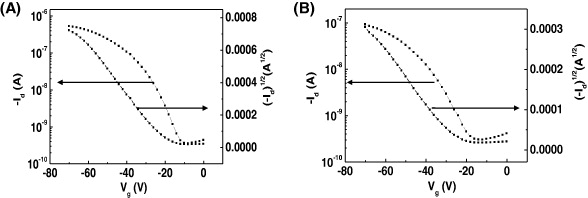
Figure 8 shows transfer curves of OFET made by spin coating the monochlorobenzene solution of (A) HHBT and (B) HDBT. The transistor devices made of HHBT and HDBT on the OTS treated SiO 2 provided field effect average mobilities of 1.4×10−4 and 2.8×10−5 cm 2 V −1 s −1, respectively, together with high current on/off ratios and relatively low threshold voltages (V th < –15 V). The mobilities and current on/off ratios were observed to strongly depend on the annealing temperature of 170 °C for HHBT and 140 °C for HDBT, and they seem to be suitable as an annealing temperature. The device fabricated with HHBT exhibited higher carrier mobility than HDBT, which might be due to a different alkyl group at the periphery. Alkyl chains have a high dielectric nature and too long an alkyl chain is not good for carrier transportation. The average mobility values obtained from the measurement of different devices are listed in table 2.
Figure 8 Transfer curves of OFET made by spin coating the monochlorobenzene solution of (A) HHBT and (B) HDBT.
4. Conclusion
We have successfully synthesized and characterized new semiconducting materials, based on a triphenylene core, which are solution processable. HHBT and HDBT have good solubility and are applicable to soluble processing for OFET device fabrication. The other molecule, HDTPT, is a new discotic liquid crystalline semiconducting material. This molecule is readily soluble in common organic solvents to exhibit self-organizing long fiber structures with supramolecularly ordered columnar stacks that lie parallel to the substrate. Highly ordered columnar structures were also built up by alternating HTMT molecules and HHBT or HDBT molecules. Among triphenylene derivatives reported in the literature, these molecules showed lower optical bandgap energies. As far as we know, this is the first time that π-extended triphenylene molecules have been reported in OFET fabrication. Although their mobilities were not so high, this work will contribute to the design of new triphenylene derivatives for future electronic applications.
Acknowledgments
This research work was supported by the Korea Research Foundation (KRF-2007-313-C00499, 2008–2009) and the Vietnam National Foundation for Science and Technology Development (NAFOSTED-104.04.28.09). In particular, Dr Mai Ha Hoang acknowledges the financial support from the Institute of Chemistry, Vietnam Academy of Science and Technology.