Abstract
Single-walled carbon nanotubes (SWCNTs) have been successfully synthesized by chemical vapor deposition (CVD) using acetylene (C2H2) gas as a carbon source and a mixture of Fe/Mo/Co on an Al2O3 support as a catalyst. The effects of the weight percentage (wt%) of metals in the Fe/Mo/Co/Al2O3 catalysts, growth time, gas flow rate and growth temperature on SWCNT growth were studied in detail. The optimum growth conditions were found to be a growth time of 60 min, a growth temperature of 750 °C, Ar/H2/C2H2 flow rates of 420/100/14 sccm and a catalyst composition of Fe/Mo/Co/Al2O3=5/3/1/80 (wt%). The morphologies and structures of the grown SWCNTs were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and Raman spectroscopy techniques.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Much effort has been made to develop technical and viable methods for synthesizing carbon nanotubes (CNTs) that have exhibited a wealth of fascinating electrical, optical and mechanical properties with a broad range of applications [1]. Currently, there are three major methods for synthesizing CNTs: arc discharge [2], laser ablation [3] and chemical vapor deposition (CVD) [4, 5]. Among these methods, CVD is the best to produce CNTs on a large scale at low cost [6–15]. Bimetal supported catalysts, such as Ni–Fe/Al 2 O 3 [8], Co–Mo/SiO 2 [9, 11], Co–Mo/MgO [12, 13], Fe-Co/MgO [14, 15] and Fe–Mo/Al 2 O 3 [16] catalysts, were found to be effective in synthesizing CNTs with remarkable yields. Among the bimetallic catalysts, Mo containing catalysts showed better performance for single-walled carbon nanotube (SWCNT) production. From the above referred works, it can be realized that the ratio of SWCNTs and multi-walled carbon nanotubes (MWCNTs) can be controlled by changing many factors, such as the support and catalyst preparation technique, the composition of the catalysts, the system of CVD operation conditions and carbon source gases in the CVD process.
In this work, we prepared a series of Fe–Mo–Co/Al 2 O 3 catalysts with varying weight percentages of metals (wt%). The growth capability of the SWCNTs from the decomposition of acetylene gas at around 750 °C under different conditions was investigated.
2. Experimental
2.1. Preparation of supported catalysts
Fe–Mo–Co/Al 2 O 3 catalysts were prepared by a conventional impregnation method with a varying weight percentage of metals (wt%). Fe(NO 3)3.9H 2 O, (CH 3 COO)2 Co.4H 2 O and (NH 4)6 Mo 7 O 24.4H 2 O were used as the metal sources. Aluminum oxide powder (Al 2 O 3) was used as the catalyst support. In all cases, the metal salts were dissolved in ethanol by stirring, then a salt solution was impregnated with the support powder Al 2 O 3. The mixture was sonicated and then dried at 100 °C for 12 h to evaporate the ethanol. Subsequently, the product was calcined in air at a temperature of 400 °C for 30 min. The calcined catalyst was ground to sizes of 50–300 nm.
2.2. CVD process
Synthesis of the SWCNTs was carried out at atmospheric pressure via catalytic decomposition of C2H2. Approximately 20 mg of a catalyst sample was uniformly dispersed on a quartz board, which was placed in the center region of a horizontal quartz tubular reactor. The diameter of the quartz tubular reactor was 20 mm. An argon flow of 420 sccm was supplied in the whole CVD process. A hydrogen flow of 100 sccm was introduced to deoxidize the catalyst. After 10 min, acetylene (C 2 H 2) flow with varying flow rates was added to the CVD process. The growth time was 30, 60 and 90 min. After the CVD process, the furnace was cooled down to room temperature in Ar gas ambient to prevent oxidation of the CNTs.
The structure and morphology of the synthesized CNTs were characterized by using SEM, TEM and Raman spectroscopy. Raman scattering was used to characterize the CNTs with an excitation wavelength of 633 nm at room temperature.
3. Results and discussion
3.1. Effect of growth temperature
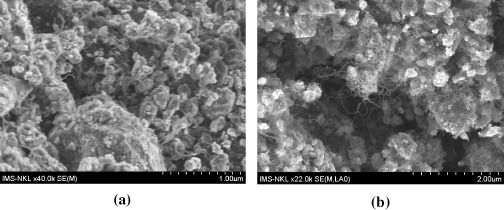
Figure 1 shows SEM images of the CNTs synthesized for 60 min over the composition Fe/Mo/Co/Al 2 O 3=5/1/1/80 (wt%) with flow rates of Ar/H 2/C 2 H 2=420/100/14 sccm at temperatures of 600, 750 and 800 °C. It is clear that the density of the CNTs was increased with increasing growth temperature. The density of CNTs grown at 600 °C (figure 1(a)) was very low and lower than that grown at 750 °C (figure 1(b)) and 800 °C (figure 1(c)). This result suggests that more active nucleation sites for the growth of CNTs could be formed at higher temperatures, resulting in a higher density of CNTs grown at 800 °C compared to those grown at 600 and 750 °C. Another way, at low temperature 600 °C, the C 2 H 2 gas is not entirely decomposed, so the carbon source for the growth process of the CNTs was very low. When the temperature was increased to 750 °C, C 2 H 2 gas could be decomposed completely. Thus, the density of the CNTs grown at 750 and 800 °C was greater than that grown at 600 °C.
Figure 1 SEM images of the CNTs grown over a Fe/Mo/Co/Al 2 O 3=5/1/1/80 (wt%) catalyst for 60 min at (a) 600 °C, (b) 750 °C and (c) 800 °C with flow rates of Ar:H 2 :C 2 H 2=420:100:14 sccm.
It was found that there were a lot of amorphous carbons on the surface of the CNTs grown at 800 °C and on the other hand, the diameters of the CNTs were larger compared to those grown at 750 °C. These results can be explained as follows. When the temperature is higher, the rate of hydrocarbon gas decomposition increases, and the rate of transportation and diffusion of carbon into the catalyst also increases. The diameter of the CNTs is therefore larger and the CNTs are made dirtier by amorphous carbons that cover the surface of the CNTs.
3.2. Effect of the growth time
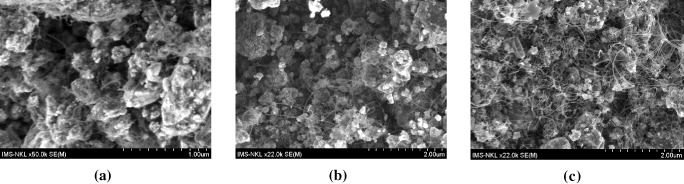
To study the influence of the growth time on the synthesis of the SWCNTs, we executed the CVD process for 30, 60 and 90 min at the same temperature and gas flow (750 °C and 420 : 100 : 14 sccm Ar:H 2 :C 2 H 2 flow rates). Figure 2 shows that when the growth time was longer, the density of the CNTs was more dense at the same temperature and gas flow. We can see that with a growth time of 30 min, the density of the CNTs was less than that for 60 min. And for 90 min, the surface of the CNTs showed a lot of amorphous carbon.
Figure 2 SEM images of the CNTs grown during (a) 30 min and (b) 60 min at 750 °C under Ar:H 2 :C 2 H 2 (420 : 100 : 14 sccm) using a catalyst of Fe/Mo/Co/Al 2 O 3 (5/1/1/80 (wt%)).
It seems that the growth speed of the CNTs depends on the catalyst conditions. In the initial period, the catalyst is pure, CNTs can grow very fast, and the density of the CNTs is high. Gradually, the catalytic particles are polluted, the catalytic activation decreases and so CNT growth slows down. The excess decomposited carbons are deposited on the surface of the CNTs to form amorphous carbon.
3.3. Effect of gas source flow rates
Figure 3 shows SEM images of the CNTs grown on the catalyst Fe/Mo/Co/Al 2 O 3=5/4/1/80 (wt%) for 60 min at 750 °C with C 2 H 2 gas flows of (a) 7 sccm, (b) 14 sccm and (c) 30 sccm, respectively.
Figure 3 SEM images of the CNTs grown over Fe/Mo/Co/Al 2 O 3=5/4/1/80 (wt%) catalyst for 60 min at 750 °C with Ar:H 2=420:100 sccm, and (a) 7 sccm, (b) 14 sccm and (c) 30 sccm C 2 H 2 flow rates.
The SEM results show that the CNT growth at a 14 sccm C 2 H 2 flow rate has higher purity compared to other flow rates.
3.4. Effect of catalyst weight percentage (wt%)
Recently, many papers have studied the influence of Mo on the synthesis process of SWCNTs with a catalyst mixture of Fe, Co, Ni, etc. or other catalysts, such as Al 2 O 3, MgO, etc. In this paper, we expose the results in investigating the role of metallic weight percentage in mixed catalysts (Fe, Mo, Co) that are covered on Al 2 O 3 particles. The influence of Mo on the SWCNT synthesis process is also studied in detail.
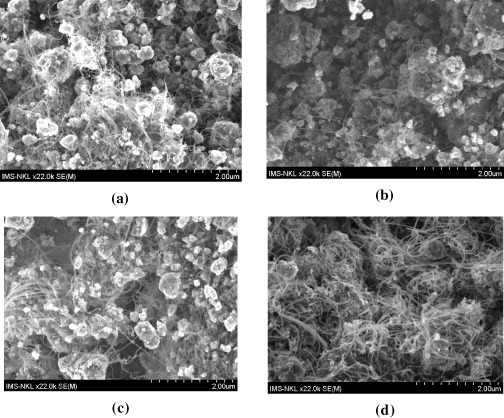
Figure 4 shows SEM images of CNTs synthesized over Fe/Mo/Co/Al 2 O 3 catalysts with varying (wt%) metals, namely: A of Fe/Mo/Co/Al 2 O 3=5/3/1/80, B of Fe/Mo/Co/Al 2 O 3=5/4/1/80, C of Fe/Mo/Co/Al 2 O 3=5/1/1/80 and D of Fe/Mo/Co/Al 2 O 3=7/1.5/1/30. The CVD process was carried out for 60 min at 750 °C with a flow of Ar/H 2/C 2 H 2=420/100/14 sccm mixture. It can be seen that the CNT density of the D catalyst was highest and the CNT density of the B catalyst was lowest. The CNT densities of the A and C catalysts were not as high as that of the D catalyst, but the CNT average diameter of the catalysts was smaller than that of the D catalyst.
Figure 4 SEM images of the CNTs grown during 60 min at 750 °C using a mix of Ar/H 2/C 2 H 2 (420/100/14 sccm), and (a) Fe/Mo/Co/ Al 2 O 3=5/3/1/80 (catalyst A), (b) Fe/Mo/Co/Al 2 O 3=5/4/1/80 (catalyst B), (c) Fe/Mo/Co/ Al 2 O 3=5/1/1/80 (catalyst C), (d) Fe/Mo/Co/ Al 2 O 3=7/1.5/1/30 (catalyst D).
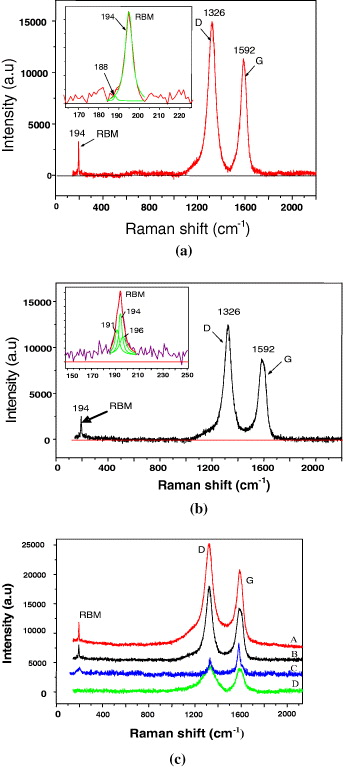
In order to understand the structure of the grown CNTs, Raman spectroscopic measurements were executed at the excitation wavelength of 633 nm. The Raman spectra of the grown CNTs at different growing conditions are shown in figure 5. It can be seen that in all cases the Raman spectra of the CNTs have two peaks: one at 1326 cm −1 (D-line) and the other at 1592 cm −1 (G-line). The G-peak originates mainly from the graphite Raman—active in plane E2g vibration mode. The D-peak is attributed to a disordered carbonaceous component. The Raman spectra of the CNTs grown over the A, B and C catalysts showed some distinct features compared with those that appeared for the D catalyst. In these cases, besides the characteristic peaks of the CNTs at 1326 and 1592 cm −1, the pronounced radial breathing mode (RBM) peaks appeared between 180 and 300 cm −1, and the intensity of the RBM peak of A catalyst was highest. This shows that Mo plays an important role in the synthesis process of SWCNTs [5, 9–12]. At the CVD temperature (600–900 °C), using gas sources (CO, C 2 H 2, CH 4), Mo does not dissolve C to create the MoC phase. Moreover, the melt temperature of Mo is higher than that of Fe and Co. So, Mo is an agent to separate catalytic metal particles and avoid agglomeration of the catalyst. Thus, the diameter of the particles is very small and is advantageous for the growth of the SWCNTs. We demonstrated that the Mo component in the catalyst mixture of Fe/Mo/Co/Al 2 O 3=5/3/1/80 is the best for the SWCNT growth process. From the measured ω RBM value and using the relationship for SWCNT diameter d(nm)=248/ω RBM [17, 18], where ω RBM is the frequency of the RBM in cm −1, the grown SWCNTs with diameter distribution ranging from 1.2 to 1.4 nm were evaluated as shown in table 1.
Figure 5 Raman spectra of the CNTs grown over (a) Fe/Mo/Co/Al 2 O 3=5/3/1/80, (b) Fe/Mo/Co/Al 2 O 3=5/4/1/80 and (c) Raman spectra of the CNTs grown over A, B, C and D catalysts for 60 min at 750 °C with flow rates of Ar:H 2 :C 2 H 2=420:100:14 sccm.
Table 1. Diameter values of SWCNTs synthesized over catalysts with varying wt% metal loaded in this work.
| Fe/Mo/Co/Al 2 O 3=5/1/1/80 | 176 | 1.41 |
| 199 | 1.25 | |
| Fe/Mo/Co/Al 2 O 3=5/3/1/80 | 191 | 1.30 |
| 194 | 1.28 | |
| 196 | 1.26 | |
| Fe/Mo/Co/Al 2 O 3=5/4/1/80 | 188 | 1.32 |
| 194 | 1.28 |
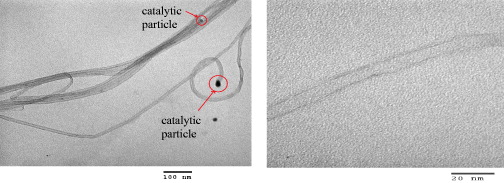
Figure 6 shows TEM images of the CNTs grown over a catalyst of Fe/Mo/Co/Al 2 O 3=5/3/1/80. It is seen that the SWCNTs created by this catalyst were formed in bunch type and their diameters were about 3–6 nm. There was no amorphous carbon on the surface of the CNTs and the catalytic particles appear at the top of the CNTs.
Figure 6 TEM images of the CNTs grown on catalysts of Fe/Mo/Co/Al 2 O 3=5/3/1/80 (wt%) for 60 min at 750 °C with flow rates of Ar:H 2 :C 2 H 2=420:100:14 sccm.
4. Conclusion
The catalyst compositions and CVD conditions for the synthesis of single-walled carbon nanotubes from C 2 H 2 decomposition have been systematically investigated in order to maximize the selectivity towards SWCNTs by chemical vapor deposition. Single-walled carbon nanotubes were successfully synthesized over Fe/Mo/Co/Al 2 O 3=5/3/1/80 (wt%) catalysts for 60 min at 750 °C at flow rates of Ar:H 2 :C 2 H 2=420:100:14 sccm. From the Raman and TEM studies, the average diameter of synthesized SWCNTs was determined to be from 1.2 to 5 nm.
Acknowledgments
We would like to thank the Research and Development of Technology program, Vietnam Academy of Science and Technology (VAST) for support. Part of this work was done with the help of the National Basic Research Fund (Nafosted, code: 103.03.47.09) and the fund of AOARD 104140 Project.