Abstract
Being naturally abundant resources and having many interesting physicochemical and biological properties, chitin/chitosan have been found to be useful in many fields, especially biomedical ones. This paper describes the strategy to design multifunctional, hybrid chitosan-based nanomaterials and test them in some typical biomedical applications.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
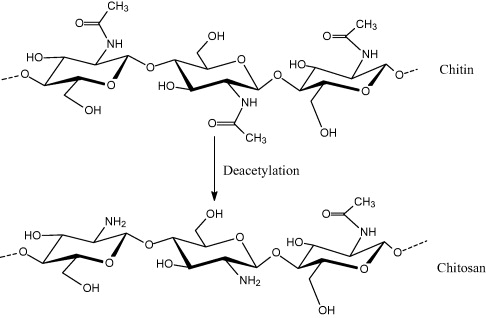
Chitin, a natural polysaccharide first identified in 1884, is the second most abundant polymer after cellulose. The most important derivative of chitin is chitosan (CS), obtained by deacetylation of chitin in the solid state under alkaline conditions, resulting in a heterogeneous distribution of acetyl groups along its chains, or by enzymatic hydrolysis in the presence of chitin deacetylase (figure 1). A wide variety of biomedical applications in tissue engineering [1,2], wound healing, drug and gene delivery [3–7] have been based on biocompatible, biodegradable and non-toxic CS. This paper demonstrates some synthetic strategies and the most important biomedical applications of chitosan-based nanoparticles and nanocomposites.
Figure 1 The structure of chitin and chitosan.
2. Materials and methods
2.1. Antibacterial and antiproliferative tests
2.1.1. Antimicrobial assays
Two bacterial strains of Bacillus subtilis ATCC 6633 and Pseudomonas aeruginosa ATCC 15442 (denoted as Bs and Pa, respectively) were investigated in this study. Bacterial strains were kept at −80 °C with 5×107 colony forming units (CFU) ml −1. Pre-diluted test silver nanoparticles (AgNPs) were treated with bacterial and fungal containing solution at 5×105 CFU ml −1. The multimicrodilution assay was used to evaluate the antibacterial activity. Namely, the 96-wells test plates were incubated overnight at 37 °C and optical densities were measured at 600 nm with a TECAN Genios multifunction fluorescence, absorbance and luminescence microplate reader equipped with an ultraviolet-visible (UV-Vis) fluorescence and absorbance reader.
2.1.2. Antiproliferative tests
Cytotoxicity was determined by means of a colorimetric microculture assay (MTT assay, MTT = 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide). Cell densities of 1.5×104 for human breast cancer cell line MCF-7 (Michigan Cancer Foundation-7) were chosen in order to ensure exponential growth throughout drug exposure. After 24 h preincubation, cells were exposed to solutions of the test compounds (0.39–10 μg ml −1 of AgNPs) in culture medium for 72 h. Optical densities were measured at 600 nm with the TECAN Genios.
All the antibacterial and anti-proliferative parameters were determined triplicate.
2.2. Evaluation of the macrophage-colony stimulating factor
Human buffycoat was obtained from the National Institute of Hematology and Transfusion (Vietnam). Mononuclear cells were isolated by density gradient centrifugation using 1.077 g ml −1 Ficoll. Cells were cultured in RPMI 1640 medium (Moore et al at Roswell Park Memorial Institute) with 1 μg ml −1 of human granulocyte macrophage-colony stimulating factor (HGM-CSF), MP Biomedicals. Then 7–12-week-old Swiss mice were obtained from the National Institute of Hygiene and Epidemiology (Vietnam). Human monocytes or mouse primary peritoneal macrophages were grown for 24 h on glass coverslips. 106 cells were incubated with 0.05 mg magnetic nanoparticles (MNPs) for 2–15 h, then treated with either a cluster of differentiation 14 (CD14) antihuman antibody (Bio Legend) or actins antibody (Invitrogen) for taking laser scanning confocal microscope (LSCM) images.
2.3. Confocal microscope analysis
LSCM images with excitation light of 488 nm were collected with the use of a ZEISS 510 LSCM with 20× or 40× or 63× oil immersion objectives. The magnetic properties were measured using a physical properties measurement system (PPMS) from Quantum Design at fields ranging from –20 to 20 kOe at 25 °C, with accuracy of 10−5 emu.
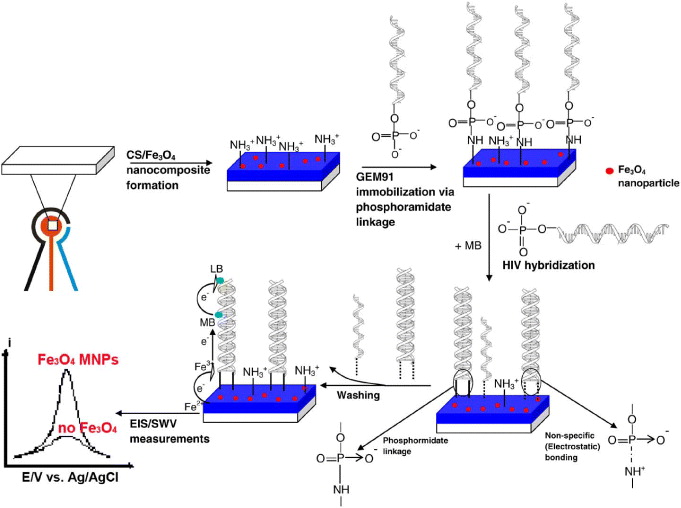
2.4. Immobilization of oligodeoxynucleotide (ODN) probe and hybridization with complementary sequences onto Fe3O4/CS screen printed electrodes (SPEs)
In order to demonstrate the applicability of ODN, the human immunodeficiency virus (HIV) sequence was chosen as the ODN model. For this purpose, 25-mer gene expression of modulator 91 (GEM 91 inhibits HIV-1 replication) was used as DNA probe (5'→3') (p-CTC TC G CAC CCA TCT CTC TCC TTC TAG). GEM probe (0.01 μM) was covalently immobilized on CS/Fe 3 O 4 via phosphoramidate reaction between the amine group of CS and phosphate group (denoted as p) at 5' terminal of the GEM sequence, in which EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, 0.03 M) and MIA (1-methyl imidazole, 0.01 M) were used to activate the amine group, with the formation of intermediate labile ester. Hybridization experiments were carried out in 1× PBS solution (pH 7) containing 0.01 μM of targets. Target sequences (5'→3') are c-HIV (A GAA GGA GAG AGA TGG GTG C), 1m-HIV (A GAA GGA GAG AAA TGG GTG C) , 2m-HIV (A GAA GAA GAG AAA TGG GTG C) and A20 (A AAA AAA AAA AAA AAA AAA A) which serve as complementary, 1-mismatch, 2-mismatch and non-complementary sequences, respectively. 1m-HIV, 2m-HIV and A20 were used in control experiments of selectivity.
3. Results and discussion
3.1. Antibacterial and proliferative activities of silver/chitosan nanoparticles
Effectively, different mechanisms for bacterial action of AgNPs are the following: (i) Ag + ions are supposed to bind to sulfhydryl groups, which lead to protein denaturation by the reduction of disulfide bonds; (ii) Ag + can form a complex with electron donor groups containing sulfur, oxygen or nitrogen that are normally present as thiol or phosphate on amino acids and nucleic acids. Also, AgNPs have been found to attach to the surface of the cell membrane and disturb its function, penetrate bacteria and release Ag +; (iii) AgNPs target the bacterial membrane, leading to dissipation of the proton motive force. Thus, a decrease in NP size can lead to an increase of the specific surface of a bactericidal specimen, inducing an increase of their ability to penetrate the cell membrane, and thus improving antibacterial activity. AgNPs, including ionic silver (Ag +) or metallic silver (Ag°), were shown to have good antibacterial and proliferative activities. Numerous methods have been proposed and reported in the literature [8–11]. In our previous studies, AgNPs were prepared by reducing silver from silver salt solutions using chitosan as both reducing and stabilizing agent [12,13]. It can be clearly observed that a core-shell structure of AgNPs/CS was successfully obtained (the composite diameter was about 12 nm, whereas the structural coating shell of chitosan was about 2 nm). The investigation of AgNPs/CS into antibacterial activities was conducted with two bacterial lines (Bs and Pa) using the multimicrodilution assay [13]. The bactericidal effects of AgNPs/CS samples (synthesized at 60, 85 and 90 °C) against Bs and Pa strains are obviously significant on control of the strains' growth. It can also be noted that the half maximal inhibitory concentration (IC 50), the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) values of the aforementioned samples against bacteria and fungi lines were achieved as promising. AgNPs/CS exerted a bactericidal effect against all bacterial strains of negative gram (Salmonella enterica, Escherichia coli and P. aeruginosa), positive gram (Lactobacillus fermentum, Staphylococcus aureus and B. subtilis) and yeast (Candida albicans) [13]. To the best of our understanding, we have achieved a surprisingly low number (1.5–6 g ml −1, except for S. enterica) with respect to IC 50, in comparison to the best result recently reported in the literature, which studied 25 nm AgNPs using Maltose as the reducing agent [9].
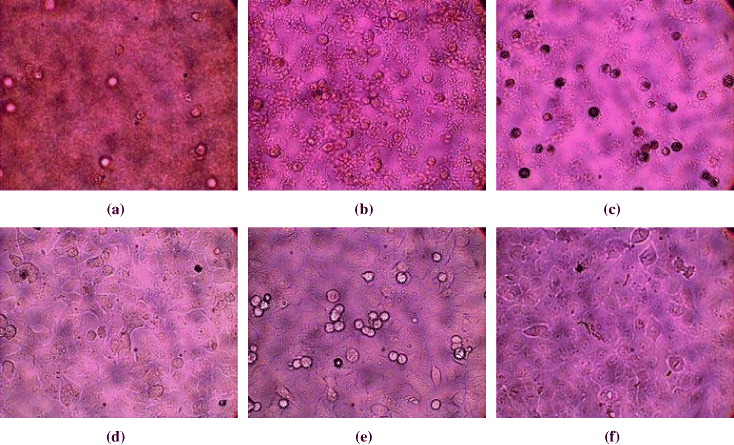
It is also known that AgNPs have gained much popularity owing to the broad spectrum of antimicrobial activity. Silver impregnated catheters and wound dressings are two main therapeutic applications. However, in spite of the wide usage of AgNPs in wound dressings, not many reports on the cytotoxicity of AgNPs are available. Therefore, in this study, assay was used to assess the effect of AgNPs on the proliferation of cancer cell lines. The cytotoxicity of AgNPs was evaluated in vitro against MCF-7 cell lines at different concentrations. The analyses showed a direct dose-response relationship, i.e. cytotoxicity increases with concentration. The tested samples demonstrated considerable cytotoxicity against the aforementioned cell lines: AgNP concentrations necessary to produce 50% cell death (IC 50) were 5.71 μg ml −1 for MCF-7 cell lines. The cellular changes upon exposure to AgNPs were also monitored (figure 2). This figure represents the morphological changes of cells during the treatment with AgNPs/CS after a period of 72 h. As illustrated in this figure, the presence of at least 6.25 μg ml −1 AgNPs significantly inhibited the cell line's growth more than 60% (figures 2(a)–(c)); at the lowest concentrations (0.39 and 1.56 μg ml −1), the cells' proliferation of all the aforementioned lines was kept intact (figures 2(d) and (e)): AgNPs were able to inhibit the cell line growth by less than 10–15%. Briefly, this result has demonstrated that AgNPs/CS remarkably inhibited the growth of MCF-7, HepG2 (human hepatocellular carcinoma), Lu (human lung carcinoma), and KB (human epidermic carcinoma) cancer lines, although their inhibitory effects differed from one to another [12].
Figure 2 Micrographs of morphological changes for MCF-7 cell lines in response to different AgNP concentrations during 72 h: (a) 100 μg ml −1; (b) 25 μg ml −1; (c) 6.25 μg ml −1; (d) 1.56 μg ml −1; (e) 0.39 μg ml −1; (f) 0 μg ml −1 (dimethyl sulfoxide solvent only) [12]. Reprinted with permission from [12], copyright 2010 Elsevier.
3.2. Drug delivery of a CS-based system
The unique physicochemical and biological characteristics of CS make it suitable for drug delivery systems. A strategy to design a multifunctional, nanosized magnetofluorescent water-dispersible Fe 3 O 4-curcumin conjugate (diameter less than 500 nm) and its multiple ability to label, target and treat the tumor cells was developed. The conjugate possesses a magnetic Fe 3 O 4 nanocore, CS as the outer shell and entrapped curcumin (Cur, an anti-oxidant, anti-inflammatory and anti-tumor substance), serving the dual function of naturally autofluorescent dye as well as antitumor model drug, delivered to the cells with the help of macrophage whose role is to phagocytise (engulf and then digest) cellular debris and pathogens, either as stationary or as mobile cells, and to stimulate lymphocytes and other immune cells to respond to the pathogen. Primary peritoneal macrophage isolation is described in detail elsewhere [14]. Hence, it can be used as a potential vehicle for transport of MNPs into the core of tumor cells.
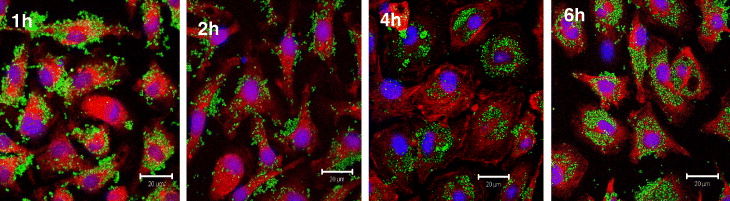
Fe 3 O 4-Cur conjugate could be visualized dually by a fluorescence microscope, LSCM as well as magnetization measurement (Physical Properties Measurement Systems, PPMS). LCSM images (figure 3), taken at 1, 2, 4 and 6 h of incubation, showed that the number of Fe 3 O 4-Cur taken into macrophage cytoplasm increased clearly with incubation time [14]. The green fluorescence was observed clearly, started at about 0.5–1 h and reaching its maximal at 6 h, confirming that the Fe 3 O 4-Cur particles were efficiently loaded. Alternatively, PPMS magnetization curves of macrophage samples at 1, 2, 4 and 6 h of incubation (figure not shown) also confirmed that the magnetization of macrophage increases (magnetization of the remaining supernatants decreases). This magnetization result is, therefore, in good accordance with that achieved by the above-demonstrated in-situ observation by LSCM fluorescence [14].
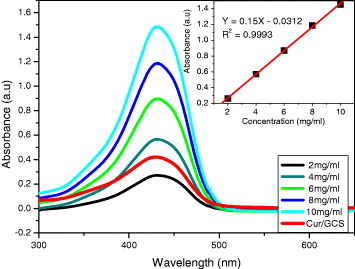
Further, the excellent loading and releasing capacity of Cur from Gossypol/CS carrier was clearly demonstrated in figures 4 and 5, respectively. The successful development of an effective drug nanocarrier for other therapeutic treatments can be logically expected.
Figure 4 The solubility of Cur in aqueous solutions without (left) and with (right) Gossypol/CS nanocarrier.
Figure 5 The relationship between absorbance and wavelength at different concentrations of Cur, released from Gossypol/CS nanocarrier.
3.3. Magnetic inductive heating capacity of the ferrofluids
Hyperthermia is a promising approach to cancer therapy. It is well-known that MNPs exhibit a so-called magnetic heating (MH) effect—a very specific property that has attracted much research interest recently because of its potential applications in cancer hyperthermia, drug release and also the desorption of toxic substances [15,16]. However, to be applicable in such applications, Fe 3 O 4 has to be encapsulated to avoid agglomeration or to make monodisperse in suspension. Therefore it is essential to modify the surface of these particles to increase the stability by surfactants, among which biocompatible polymers like CS and its derivatives, e.g. O-carboxymethylchitosan (OCMCS), are the most potential candidates owing to their biocompatibility and biodegradability [13].
3.4. Electrochemical detection of ODN sequences on CS/Fe3O4 SPEs
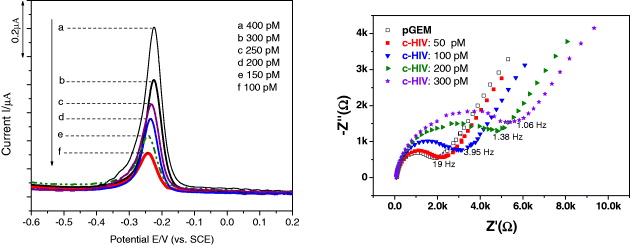
A selective and sensitive response to mismatch is always among the most difficult technical challenges of any electrochemical biosensor [17–19]. The whole procedure of HIV detection is schematically presented in figure 6 [20]. The effective discrimination against one point mutation of 1m-HIV was systematically carried out. As expected, there was no significant current difference observed for the GEM91-modified SPEs when they were hybridized with either non-complementary or single base mismatch 1m-HIV sequences. Hybridization-induced signal decrease occurs on the electrode platform where GEM probes were covalently fixed on the surface and were subsequently hybridized with truly complementary c-HIV sequences. Meanwhile, there was no signal change during hybridization with 1m-HIV, whose strand was differentiated by one mismatched base (adenine (A) instead of guanine (G)), compared to that of the c-HIV strand.
The square wave voltammetry (SWV) and electrochemical impedance spectroscopy-Nyquist plot (EIS-Nyquist plot) response to different target DNA concentrations is displayed in figure 7. The decrease of the magnitude of the voltammetric signal thus reflected the extent of the hybrid formation. The signal for the hybridization with c-HIV is linear from 50 pM up to approximately 300 pM of c-HIV target, and levels-off beyond this concentration limit, indicating that the full surface coverage of the probe or GEM 91 was involved in hybridization at that target concentration (probe site saturation was achieved). The phenomenon that the current of methylene blue (MB) decreases with increasing concentration of HIV is explained by the higher affinity of MB for a single strand rather than double strands of DNA. These results showed reasonable agreement with the common concept for MB intercalator [20].
4. Conclusion
Although not fully described/reviewed, this paper presents some useful chemical routes to functionalize hybrid chitosan-based nanomaterials and demonstrates their use in some potential biomedical applications (antibacterial and antiproliferative activities, drug delivery, biodetection). The ability of these materials was clearly observed by many physico-chemical methods. Further developments in material design/synthesis for enhanced therapeutic and diagnostic effects will be continued and reported in upcoming papers.
Acknowledgments
This work was performed under the financial support of the Ministry of Science and Technology project, code 08/2011/HD-NDT. The authors would like to express their gratitude to Academician Nguyen Van Hieu for his encouragement and to the second ASEAN-Pakistan Conference on Materials Science (APCOM2) for support.