Abstract
Perovskite-type oxides La0.9M0.1CoO3 (M=K/Na/Li) were prepared by a citrate sol–gel method. These oxides were characterized by x-ray diffraction analysis (XRD), a scanning electron microscope (SEM) and the N2 physisorption Brunauer–Emmitt–Teller (BET) method. Their catalytic activity was studied by a constant microflow method for CO- and C3H6-oxidation and by thermal decomposition of diesel soot. The results show that the obtained perovskite materials have a sphere shape, particles size of about 40–50 nm and surface area from 12.0 to 14.9 m2 g−1. They have high catalytic activities decreasing in the sequence K > Na > Li on conversion CO, C3H6 and diesel soot. The total conversion temperature of CO, C3H6 and diesel soot is 325 °C, 340 °C and 430 °C, respectively. Thus, these catalysts have high applicability in the removal of exhaust gas, especially from diesel engines.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Vehicle exhausts cause environmental pollution. Exhaust gas from petrol engines can be successfully cleaned by three-way catalytic systems, while emissions from diesel engines have become an increasingly serious concern. Diesel engines combine a high fuel economy with high durability and low maintenance costs and, therefore, are used on a large scale for transportation purposes. However, diesel engines generate more NO x and carcinogenic particulates, whose size (50–20 nm) falls in the so-called lung-damaging range [1]. Many toxicological and epidemiologic studies have established adverse health effects by particulate matter (PM10, PM2.5). There is increasing evidence that several health effects are associated with ultrafine particles (diameter < 100 nm) [2, 3]. Therefore, to reduce pollution from diesel engines, both oxidation (for removal of CO, C x H y , volatile organic compounds—VOCs and diesel soot) and NO x reduction catalysts have to be used.
Perovskite-type oxides of ABO 3 (A is lanthanide and/or alkaline earth metal ion and B is transition metal ion) doping by substitution of earth alkali/alkali cations at A and B exhibit good catalytic activity [4–10]. The addition of silver into substituted perovskite and/or oxides leads to improvement of catalytic activity not only in the oxidation (of CO, VOCs, aromatic hydrocarbons), oxygen storage but also in the reactions of NO x removal at relatively low temperatures [5–7]. Several perovskites substituted by the alkali elements are promising catalysts for the efficient conversion of NO x and diesel soot into N 2, CO 2 and H 2 O, which are environmentally friendly [8–10]. When the alkali metal ions partially replace lanthanum in LaCoO 3 perovskite, the samples will have large catalytic activity for diesel soot combustion. However, if the alkali metal content increases, the ability to create single phase perovskite is more difficult. Therefore, in order to research the oxidation activity of a catalyst and compare the role of alkali metals played in the catalyst La 1-x M x CoO 3, we chose x=0.1. The La 0.9 M 0.1 CoO 3 (M=K/Na/Li) was prepared by a citrate sol–gel method. The catalytic activity of the materials was investigated in oxidation reactions of CO, C 3 H 6 (99.99%) and the combustion of diesel soot. In the experiment, CO and C 3 H 6 were purchased from Air Liquide and diesel soot was taken from Ford Ranger in Vietnam.
2. Experimental
2.1. Catalyst preparation
Reagents such as La(NO3)3, KNO3, NaNO3, Li2CO3, Co(CH3COO)2·4H 2O, C6H8O7·H 2O, NH4OH and CH3COOH were of analytical grade. A series of perovskite catalysts (La0.9K0.1CoO3, La0.9Na0.1CoO3 and La0.9Li0.1CoO3) were prepared by a citrate sol–gel method as follows: the reagents were first dissolved in twice distilled water in stoichiometric amounts. Citric acid was added with the following molar ratio metal:citrate=1:1.5. The solution was adjusted to pH = 7.5–8.0 with aqueous ammonia or acetic acid, stirred and heated to a temperature of 60–80 °C for 4 h. A dark-rose gel product was obtained. The gel was then heated to 100 °C in 24 h in air, then calcined at 450 °C for 2 h, and the temperature was raised to 600 °C in 4 h. Black samples were obtained.
2.2. Catalyst characterization
(XRD) analysis was carried out using a Siemens D5000 x-ray diffractometer with a Cu–K α cathode (λ=1.5406 Å). SEM images were obtained by a scanning electron microscope on a Hitachi S 4800 system. The BET specific surface area was measured on a Micromeritics Tristar 3000 V6.07.
2.3. Catalytic test
The catalytic activity of the materials in the oxidation reaction of carbon monoxide and propene was studied by a constant microflow method (for determining the concentration and treatment efficiency of some toxic gases). The catalytic activity of the soot decomposition was investigated by the combustion of a mixture containing catalyst and soot with a 10:1 initial mass ratio in air in 2 h.
3. Results and discussion
3.1. Identification of materials by XRD
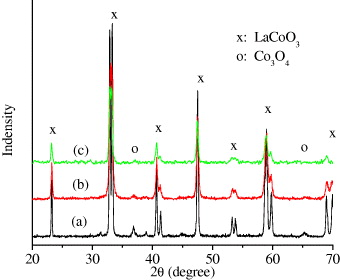
The XRD patterns of the La 0.9 M 0.1 CoO 3 (M=K/Na/Li) samples calcined at 600 °C in air for 4 h are presented in figure 1. All peaks of the La 0.9 K 0.1 CoO 3 were completely indexed as the perovskite-type structure, but the peaks of the others were indicated as a mixture of perovskite-type LaCoO 3 and Co 3 O 4. On the other hand, the intensity of the Co 3 O 4 peaks in figure 1(a) is higher than that in figure 1(b). It means that the ability to form perovskite single phase decreases in the ranking K–Na–Li when substituting them into LaCoO3. It is similar to the result of Wang et al [9] when they studied the catalytic behavior of La–Mn–O (doping by Na/K/Li) nanoparticle perovskite-type oxide catalysts for the combustion of soot particles from a diesel engine.
Figure 1 XRD patterns of La 0.9 Li 0.1 CoO 3 (a), La 0.9 Na 0.1 CoO 3 (b) and La 0.9 K 0.1 CoO 3 (c).
3.2. SEM and BET results
Figure 2 presents the SEM images of the La 0.9 M 0.1 CoO 3 (M=K/Na/Li) samples. It indicates that most of the perovskite crystals have a sphere shape and range between 40 and 50 nm in size. However, an agglomeration phenomenon of some particles is observed.
Figure 2 SEM images of La 0.9 M 0.1 CoO 3 samples.
By a N 2 physisorption method, the BET specific surface area of the samples was determined from 12.0 to 14.9 m 2 g −1 (table 1).
Table 1. The BET specific surface area of La 0.9 M 0.1 CoO 3.
| La 0.9 K 0.1 CoO 3 | 14.896 |
| La 0.9 Na 0.1 CoO 3 | 12.036 |
| La 0.9 Li 0.1 CoO 3 | 12.970 |
| La 0.9 K 0.1 CoO 3 [10] | 2.569 |
The obtained results showed that the specific surface area (BET) of the sample containing potassium is higher than that with lithium and sodium. According to [10], when La 0.9 K 0.1 CoO 3 is calcined at 1050 °C, the BET reduces to 2.569 m 2 g −1. Thus for production technology it is necessary to decrease the calcination temperature to obtain a high specific surface area as well as high oxidation activity catalytic.
3.3. Catalytic properties in the oxidation reactions
3.3.1. Catalytic activity in CO-oxidation
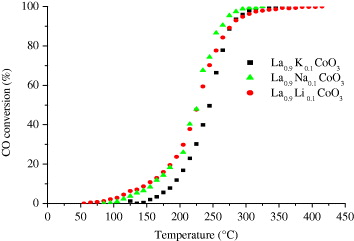
Figure 3 shows the dependence of CO-conversion on the reaction temperature over catalyst La 0.9 M 0.1 CoO 3 (M=K/Na/Li). These complex oxides exhibit rather high catalytic activity at a relatively low temperature. From figure 3, three specific temperatures of the samples are obtained—as T ig , T 50 and T f —and given in table 2.
Figure 3 Dependence of CO-conversion on the reaction temperature over catalysts La 0.9 M 0.1 CoO 3 (M=K/Na/Li).
Table 2. CO oxidation temperature of the La 0.9 M 0.1 CoO 3 system (M=K/Na/Li).
| La 0.9 K 0.1 CoO 3 | 70 | 226 | 325 |
| La 0.9 Na 0.1 CoO 3 | 40 | 227 | 415 |
| La 0.9 Li 0.1 CoO 3 | 120 | 245 | 335 |
Obviously, each alkali metal expresses its own ability. The Na-doped sample has the lowest T ig -temperature (40 °C)—approximately room temperature, following by the K- and Li-doped samples (70 °C and 120 °C, respectively). The T50 value of the Na-doped sample is the same as the K-doped sample (227/226 o C) and both of these values are 20 °C lower than the Li-doped sample (245 °C), meaning T50,K ≈T50,Na <T50,Li . All catalysts are able to completely converse carbon monoxide and T f,K <T f,Li <T f,Na . These data indicate that the catalytic activity of La 0.9 K 0.1 CoO 3 can be considered as the best but the La 0.9 Na 0.1 CoO 3 catalyst seems more convenient for treatment of CO because its ignition temperature is relatively low—approximately ambient temperature.
3.3.2. Catalytic activity in C3H6-oxidation
Previous research has found that the substitution of alkali metals for lanthanum in LaCoO3 would improve its catalytic activity [8–12]. Our experiments obtained the following results.
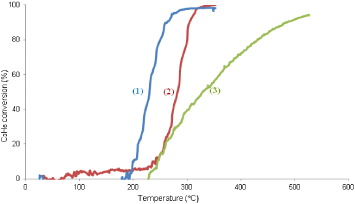
Figure 4 shows the dependence of C 3 H 6-conversion on the reaction temperature over La 0.9 M 0.1 CoO 3 (M=K/Na/Li) systems. The specific temperatures (T ig , T50, T f ) for C 3 H 6 conversion of the samples are given in table 3.
Figure 4 Dependence of C 3 H 6-conversion on the reaction temperature over La 0.9 M 0.1 CoO 3 (M=K/Na/Li) systems: (1) La 0.9 K 0.1 CoO 3,(2) La 0.9 Na 0.1 CoO 3 and (3) La 0.9 Li 0.1 CoO 3.
Table 3. C 3 H 6 oxidation temperature of the La 0.9 M 0.1 CoO 3 system (M=K/Na/Li).
| La 0.9 K 0.1 CoO 3 | 70 | 230 | 340 |
| La 0.9 Na 0.1 CoO 3 | 87 | 282 | 352 |
| La 0.9 Li 0.1 CoO 3 | 96 | 316 | 527 |
| LaCoO 3 [12] | 180 | 300 | 380 |
Figure 4 and table 3 show that by doping a small amount of potassium or sodium ions in LaCoO 3, the activity of catalysts increases remarkably. T ig , T50 and T f values of substituted LaCoO 3 are much lower than that of pure LaCoO 3. To explain this modification we propose that when doping alkali metals on perovskite, there is more lattice distortion. Moreover, the more La 3+ cations are constituted by M + cations, the greater the lack of charge. To balance the charge in the crystals, some of the Co 3+ ions have to convert into Co 4+ ions. It is known that Co 4+ ions have higher oxidation activity than Co 3+ ions. On the other hand, the reaction rate of the catalyst is dependent on adsorption, reaction and desorption processes. In the sample La 0.9 K 0.1 CoO 3, the ionic radius of potassium (152 pm) is larger than that of lanthanum (117 pm), so the oxygen adsorpted on the surface of the catalyst could be desorbed more easily and the desorption process is faster. The more the desorption process rate increases, the more the catalytic activity improves. However, all the reasons to clarify this problem are not complete. It needs wider and deeper study.
3.3.3. Diesel soot combustion
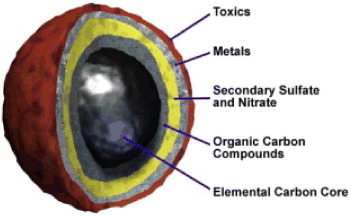
The diesel soot was made from solid particles with a size of about 0.3 μm. It is a complex mixture consisting of incomplete combustion carbon, hydrocarbons (C x H y ), CO and other compounds adsorbed on the surface as shown in figure 5.
Figure 5 Black carbon makes up the core of a diesel particle [3].
The results mentioned above about CO and C 3 H 6 conversions suggest their application to diesel soot combustion. In the following study, the effect of temperature on diesel soot combustion of La 0.9 K 0.1 CoO 3 and La 0.9 Na 0.1 CoO 3 catalysts is investigated. Soot and catalyst are taken in a 1:10 ratio of weight.
Figure 6 shows the dependence on reaction temperature of the soot conversion (α ds ) with the use of catalysts La 0.9 K 0.1 CoO 3 (curve 1) and La 0.9 Na 0.1 CoO 3 (curve 2) and without using a catalyst (curve 3). Under 250 °C, α ds values of the three samples are almost equal. From 250 to 300 °C, the effect of these catalysts on soot combustion is observed but there is no difference between them. Over 300 °C, α ds values of the catalyst-containing samples are much higher than those of the case without catalyst and α ds,La 0.9 K 0.1 CoO 3 > α ds,La 0.9 Na 0.1 CoO 3 . The complete conversion temperature over the sample doping K is lower than that with Na (430 and 450 °C, respectively). Thus the K-doped catalyst is better than the Na-doped catalyst. The soot could be completely decomposed over these catalysts at 450 °C, while over the sample without catalyst the soot conversion has a maximum value of only about 62% at 700 °C. From the results mentioned above we can see that the T50 and T100 (50 and 100% conversion temperature) of diesel soot (from Ford Ranger in Vietnam) over our obtained catalysts are about 340 and 430 °C, respectively. It can be compared with the results of Guizhen et al [13] in the case of using diesel soot of Printex-U mixed with ethanol. These authors indicated that T50 and T90 (90%-conversion temperature) values are 336 and 359 °C, respectively (the BET surface area of their material is 11.7 m 2 g −1, slightly smaller than ours—14.89 m 2 g −1).
Figure 6 The dependence of diesel soot combustion on temperature over the samples: (1) La 0.9 K 0.1 CoO 3 catalyst, (2) La 0.9 Na 0.1 CoO 3 catalyst and (3) without catalyst.
4. Conclusions
Three kinds of catalysts (La 0.9 M 0.1 CoO 3 where M=K/Na/Li) were synthesized by a citrate sol–gel method. Most of the particles have a size ranging from 40 to 50 nm and the specific surface area (BET) of the samples is from 12.0 to 14.9 m 2 g −1. The ability to form perovskite single phase decreases in the ranking K-Na-Li when substituting these metals into LaCoO 3.
Potassium shows better promotion of catalytic activity than sodium and lithium when La 3+ is substituted by these ions. The total conversion temperature of CO, C 3 H 6 and diesel soot of the K-doped catalyst is quite low, 325, 340 and 430 °C, respectively.
The obtained catalysts, which are inexpensive and have high activity in the low temperature, could be applied in environmental technology for exhaust gas removal, especially from diesel engines.
Acknowledgments
This work was supported by Vietnam's National Foundation for Science and Technology Development, no. 103.99-2010.25.