Abstract
Clear and uniform one-dimensional (1D) α-MnO2 nanorods with diameters of 30–50 nm were prepared by hydrothermal treatment of homogeneous solution of hydrated MnSO4 and KMnO4 with a little H2O2. Upon control of hydrothermal conditions (reaction temperature and duration time), an excellent crystalline phase of MnO2 was obtained and its morphology was investigated. The crystal structures of as-synthesized MnO2 products have been characterized by x-ray diffraction (XRD). The morphology of the product was examined by scanning electron microscopy (SEM) and the chemical composition was analyzed using energy dispersive spectroscopy (EDS) along with SEM. The Mn concentration was determined by the chemical titration method.

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
A wide variety of synthetic approaches have been developed for the synthesis of MnO 2 nanostructures including nanorods, nanowires, nanotubes, nanofibes and nanoneedles [1–5]. It is well known that manganese dioxide exists in various polymorphic forms including α-, β-, γ- and δ-MnO 2 which are different in the arrangement of basic octahedral [MnO 6] units [2, 3]. Among these manganese dioxides, 1D α-MnO 2 nanorods have received special attention as cathodic materials for lithium batteries since the large tunnels existing in the crystalline lattice of α-MnO 2 are believed to facilitate the accommodation and transportation of inserting lithium ions [3–6].
MnO 2 nanorods are widely used as catalysts, molecular sieves and especially as electrode materials in Zn–MnO 2 batteries, Li/MnO 2 batteries and supercapacitors. Furthermore, α-MnO 2 nanorods are used for preparing nanostructured LiMn 2 O 4 cathodic materials which show better electrochemical properties than conventional cathodic materials and have a significant advantage over those of other morphologies [7].
For nanosize metal oxide powder preparation, the hydrothermal method is one of the best. There are varieties of hydrothermal processes such as oxidation, crystallization, precipitation and decomposition, etc [8]. In this paper, we report a novel and simple approach to synthesize α-MnO 2 single-crystal nanorods by hydrothermal homogeneous precipitation. The influence of parameters such as reaction temperature and duration time on the growth and morphologies of MnO 2 nanoparticles has been investigated.
The addition of hydrogen peroxide imposed two effects on the system: (i) as a reductant it reduces KMnO 4 to MnO 2 and (ii) as an oxidant it oxidizes MnSO 4 to MnO 2 [9, 10]. The high homogeneity of precursor solutions determines low temperatures of heat treatment, which makes it possible to obtain an oxide material in nanocrystalline form.
2. Experimental procedures
All the chemical reagents were of analytical grade and used without purification. The synthesis of α-MnO 2 nanorods was divided into two steps: preparation of precursor solution (homogeneous clear solution) and hydrothermal treatment of the mixed solution by an autoclave reactor at low temperature.
In a typical system, 3.16 g (0.02 mole) of KMnO 4 and 1.69 g (0.01 mole) of MnSO 4·H 2 O were mixed and dissolved at room temperature in 70 ml of H 2 O 2 solution (1:1 molar ratios of H 2 O 2 to distilled water) by magnetic stirring to form a homogeneous solution. When the mixed solution changed to a dark brown gel-like solution, it was immediately transferred to the autoclave, sealed and heated at 70–180 °C for 2 h. After the reaction was complete, the reactor was taken out and naturally cooled to room temperature. The resulting brown-black precipitates were filtered off, washed with distilled water several times to remove excess ions, and finally dried at 120 °C in air overnight.
Several experiments were performed by varying the reaction temperature (70, 80, 90, 100, 120, 140, 160 and 180 °C) and the duration time (2, 3 and 4 h). The samples were collected at different temperatures and duration times.
The crystal structures of these various samples were characterized by x-ray diffraction (XRD) (D8 Advance Bruker Co. diffractometer operating in the reflection mode at CuKα radiation). The morphology of the samples was also investigated using scanning electron microscopy (SEM) (EVO @ 60 Carizeiss). The chemical compositions of the as-synthesized products were analyzed by energy dispersive spectroscopy (EDS) along with SEM. In addition, a chemical titration method was used to confirm the amount of Mn in the as-synthesized products as well as in the starting materials.
3. Results and discussion
All the hydrothermally as-synthesized samples obtained from various experiments were characterized by XRD. The sample having all diffraction peaks which perfectly indexed to the tetragonal α-MnO 2 single-crystal structure (JCPDS 044-0141) of the standard card was the result of excellent crystal growth of the α-MnO 2 nanoparticles.
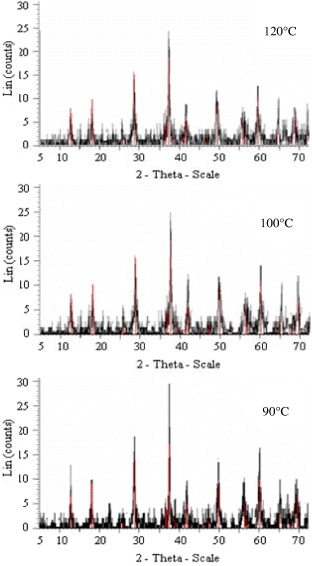
Figure 1 shows the XRD pattern of the resulting MnO 2 product. It is known that α-MnO 2 has a tunnel structure, generally with some large stabilizing cation (such as K +, Ba 2+, Pb 2+ or NH 4 +) embedded in its tunnel cavity. So a small amount of K, possibly introduced from KMnO 4 in the reaction course, was located within the tunnel of α-MnO 2 [11].
Figure 1 XRD pattern of α-MnO 2 products synthesized at different temperatures (90, 100 and 120 °C) for 2 h.
It was found that the optimal results on crystal growth of α-MnO 2 nanoparticles were obtained in homogeneous solution at a temperature of 90 °C for 2 h. The pressure in the reactor was monitored by an optical pressure transducer (the accuracy of determination of pressure at 90 °C is 8.87 atm).
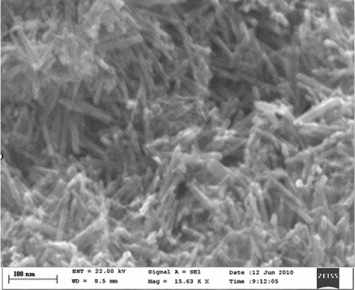
To investigate the morphology of the resulting sample with optimal reaction conditions, it was examined by SEM. Figure 2 shows SEM images of α-MnO 2 products synthesized at 90 °C for 2 h. It can be seen that they consist of rod-like nanostructures with an average diameter of 30–50 nm, an average length of 2μm, and are present in a large quantity.
Figure 2 SEM images of α-MnO 2 products synthesized at 90 °C for 2 h.
We observed that the polymorphs of MnO 2 and their morphology depend on the reaction temperature and duration. The reaction temperature plays a crucial role in controlling the growth of MnO 2. If the temperature was below 70 °C, amorphous MnO 2 particles were obtained. At 80 °C, it was found that the XRD patterns of the sample have some nondetected diffraction lines together with those of MnO 2. Above 120 °C, two polymorphs of MnO 2 were grown: α-MnO 2 (JCPDS 044-014) and β-MnO 2 (JCPDS 024-0735). Therefore, a suitable temperature for preparing α-MnO 2 nanoparticles was in the range of 90–120 °C.
The reaction time had a significant influence on the formation of the MnO 2 polymorph and played another crucial role. At this temperature range all XRD patterns of the sample products show single-phase α-MnO 2 nanoparticles. By varying the duration times, it was found that the SEM image of α-MnO 2 product at a short duration time of 2 h shows clear and uniform rod-like nanostructures. When the duration time was extended to 8 h, rod-like and wire-like nanoparticles aggregate together and bulk products or particles were obtained.
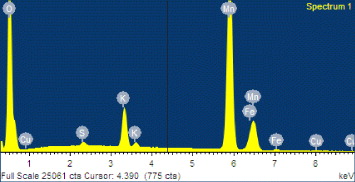
After selecting the excellent as-synthesized product, its chemical composition was analyzed by EDS (figure 3).
Figure 3 EDS analysis of as-synthesized α-MnO 2 nanorods.
From EDS analysis it was found that the product was composed of the following elements: Mn, O and K. The peak of Cu was caused by the Cu microgrid on which the product was placed. A small amount of Fe was possibly contaminated by instrumentation (autoclave). The concentrations of different elements are presented in table 1.
Table 1. Concentrations of elements in the α-MnO 2 nanorod.
ElementWeight (%)| O | 35.97 |
| S | 0.26 |
| K | 3.19 |
| Mn | 60.86 |
| Fe | 0.60 |
| Cu | 0.41 |
| Total | 100.00 |
The production yield of α-MnO 2 nanorods is above 92% based on Mn quantity in the starting reagents.
4. Conclusions
Pure and uniform α-MnO 2 nanorods were synthesized by hydrothermal homogeneous precipitation. The reaction system is a single redox system of KMnO 4 and MnSO 4·H 2 O with the addition of H 2 O 2·H 2 O 2 provides a substantial amount of oxygen for rapid redox reaction. The hydrothermal homogeneous precipitation allows for the preparation of different polymorphs of MnO 2 in different shapes by simply adjusting the reaction temperature and duration time without the need for extra reagents and additives.
Based on our experimental results we conclude that 1D α-MnO 2 nanorods with diameters of 30–50 nm and length of 2 μm are successfully synthesized by the hydrothermal treatment of homogeneous solution of KMnO 4, MnSO 4·H 2 O and H 2 O 2 with the stoichiometric ratio at 90 °C for 2 h.
Acknowledgments
This research was supported by the Ministry of Science and Technology, Myanmar. The author is thankful to the Director General who provided necessary support and valuable suggestions and also thanks the staff in the Metallurgy Department, Chemical Assay Department, XRF Department and XRD Department in the Metallurgical Research and Development Centre (Ela). The authors acknowledge Pyin Oo Lwin (Research Department) for valuable co-operation with XRD and SEM analyses.