Abstract
In this work implantable micro-probes for central nervous system (CNS) studies were developed on silicon and polyimide substrates. The probes which contained micro-electrode arrays with different surface modifications were designed for implantation in the CNS. The electrode surfaces were modified with nano-scale structures that could greatly increase the active surface area in order to enhance the electrochemical current outputs while maintaining micro-scale dimensions of the electrodes and probes. The electrodes were made of gold or platinum, and designed with different sizes. The silicon probes were modified by silicon nanowires fabricated with the vapor–liquid–solid mechanism at high temperatures. With polyimide substrates, the nanostructure modification was carried out by applying concentrated gold or silver colloid solutions onto the micro-electrodes at room temperature. The surfaces of electrodes before and after modification were observed by scanning electron microscopy. The silicon nanowire-modified surface was characterized by cyclic voltammetry. Experiments were carried out to investigate the improvement in sensing performance. The modified electrodes were tested with H2O2, electrochemical L-glutamate and dopamine. Comparisons between electrodes with and without nanostructure modification were conducted showing that the modifications have enhanced the signal outputs of the electrochemical neurotransmitter sensors.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
It is important to monitor neurotransmitters extracellularly in the central nervous system (CNS) in order to understand their roles in various neurological disorders, such as Parkinson's disease, Alzheimer's disease, depression, addiction and chronic pain. Several methods have been proposed, investigated and used to detect neurotransmitters in association with neuronal diseases, behavior and responses. Techniques utilizing micro-dialysis probes have been widely used providing great sensitivity and selectivity, but the poor temporal and spatial resolutions limit their use in real-time in vivo studies. Optical approaches are costly, bulky, complicated, and require careful calibration and specific labels. Electrochemical methods are desirable due to their fast operation, compactness and versatility. Numerous electrochemical techniques have been investigated such as amperometry, chronoamperometry, fast cyclic voltammetry and fast-scan cyclic voltammetry. Utilizing them makes it possible for scientists to obtain in vivo and real-time measurements of neurotransmitters to carry out short- or long-term studies [1–3].
Microwires made of platinum (Pt), iridium (Ir) and carbon fiber electrodes (CFE) have been conventionally used as electrode bases to detect various neurotransmitters such as L-glutamate (Glu), dopamine (DA), serotonin (5-HT) and norepinephrine (NE) [1–3]. Nevertheless, technical difficulties and challenges exist in modification of these types of electrodes with specific enzymes and/or integration of various types to correlate multiple signals [2]. With recent advances in micro-fabrication, those aforementioned issues could be addressed with planar micro-electrode arrays (MEAs). The techniques have been proven to provide advantages of multiple-target sensing and flexibility for functionalized surfaces over the conventional carbon fiber electrodes or metal wire electrodes [4–10]. However, the low output electrical current ranges have presented technical challenges in recording and interpreting the signals with cost-effective or portable electronics. This issue calls for a need to increase the signal levels, in both baseline and sensitivity [2].
We reviewed and reported the development of micro-probes containing thin-film MEAs, which could be used for electrochemical neurotransmitter sensors, based on silicon and polyimide substrates. The silicon substrates provide many conventional advantages in mass-production fabrication such as the ability to tolerate high-temperature processes, electronics integration and cost effectiveness. The flexibility of the polyimide substrates helps to prevent scar forming aiming for long-term implantations. In this work, several surface modification methods have been proposed and investigated in order to enhance the performance of the sensors based on those two substrates. Experiments have been carried out to demonstrate the capabilities of the probes and to compare for improvement.
2. Design and implementation
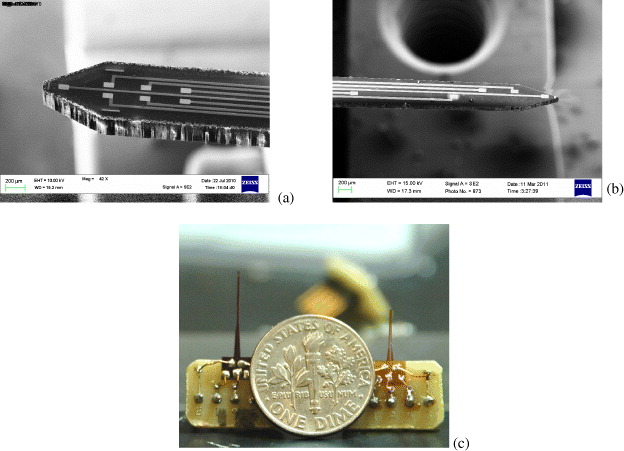
The micro-probes were fabricated monolithically in batches and tailored into individual probes by a laser micro-machining system. The details were described in [2, 3]. Printed circuit boards (PCBs) were designed to mount the probes connected with copper wires and silver epoxy. Standard connection pins were soldered onto the PCBs for assembly into a recording system. Figure 1 shows scanning electron microscope (SEM) images of the probe tips and the assembled devices.
Figure 1 (a) SEM image of the Si probe tip, (b) SEM image of the polyimide probe tip, (c) assembled devices (left: silicon, right: polyidmide) compared to a US dime.
The output current recorded in electrochemical sensors can be described by [1]

where i is the electrical current measured, n is the number of electrons for the specific chemical reaction, F is the Faraday constant of 96 500 C mol –1, A is the active surface area, C b is the bulk solution concentration of the analyte, and M is a term about mass transport of the analyte to the electrode surface. Without physically increasing the two-dimensional areas of the electrodes in order to keep them in micro-scales, the active surface area can be increased by modification with nanostructures on the surface in the third dimension, resulting in a gain of the total current output [2] owing to the increase of electrochemical reaction.
2.1. Silicon probes
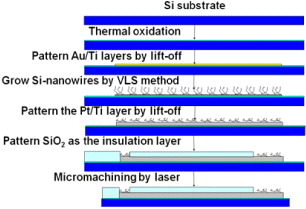
The nanowire modification in the Si probes was based on a plasma-enhanced chemical vapor deposition (PECVD) process using the vapor–liquid–solid (VLS) mechanism [2]. The VLS method developed by Wagner and Ellis in 1964 has been proved productive to grow Si nanowires. In this method, metal nano-droplets are created on a surface (Si, SiO 2, metals) and then the gas that carries the source material, which generally is silane (SiH 4) or tetrachlorosilane (SiCl 4), is introduced into the deposition chamber maintained above the eutectic temperature. The nano-droplets which are liquid will be the preferred sites for the vapor to deposit. The carrier gas then reacts to form liquid eutectic particles, as follows:
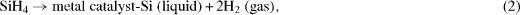
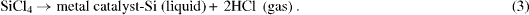
Growing nanowires on top of the electrode surface will change its effective active area and consequently increase the sensitivity in an electrochemical sensor. Furthermore, the immobilization of enzyme on working electrodes could benefit from the physical nanostructures which will play a role to enhance the enzyme adhesion on the surface of electrodes. In our processes, an initial 1 nm thick Au thin film was deposited by e-beam evaporation on an oxidized silicon wafer followed by PECVD processes under the conditions of 1100 mTorr, 420 °C and 30 sccm premixed SiH 4/Ar for 5 min. The fabrication processes utilizing the VLS technique are illustrated in figure 2.
Figure 2 Fabrication processes of the Si-nanowire modification method.
2.2. Polyimide probes
The polyimide probes provide flexibility and tissue compatibility in biomechanics. The flexibility not only enhances the biocompatibility but also provides ease and comfort in operating the devices, especially in freely moving animals. However, the polyimide substrate material can only sustain in temperatures lower than 400 °C, therefore the VLS method could not be used. An alternative method was proposed and investigated to modify the electrode surfaces with nano-scale structures at room temperature. The modification on the polyimide-based probes was done by applying concentrated gold (Au) or silver (Ag) colloid solutions onto the electrode surfaces with a Hamilton syringe under a stereo microscope. After drying, the nanostructures will stay on the electrode surfaces securely, preliminarily for several experiments. The longevity of the nano-scale structures on the electrode surface could be enhanced by a post-process to cover the electrode area with a permeable polymer film. Nafion was utilized for this purpose. The coating procedures were discussed in [2, 3].
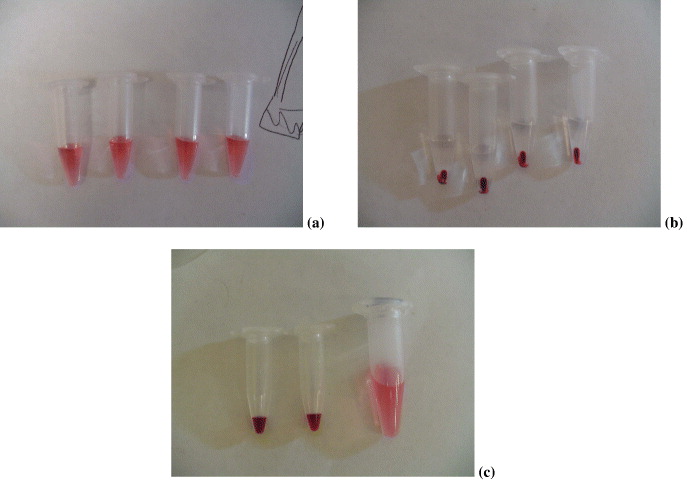
Gold colloid solutions (Ted Pella Inc.) were concentrated using a micro-centrifuge. First, original solutions were dispensed into centrifuge tubes and a centrifugation process was carried out at 7000 rpm for 5 min. The nanoparticles were concentrated in the bottom of the tubes and a certain amount of carrier solution was extracted depending on the desired target concentration. The centrifuge tubes were put into an ultrasonic bath to disperse the nanoparticles evenly. These steps were repeated in order to obtain higher concentration colloid solutions. Various concentrations in tubes are shown in figure 3. In figure 3(c), the concentrated solutions were shown to be darker than the original one.
Figure 3 Nanoparticles concentration processes. (a) original solution; (b) after centrifugation; (c) two after ultrasonication (left) compared with the original one (right).
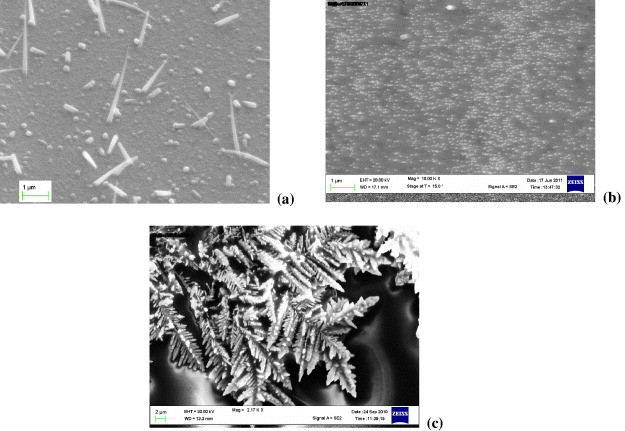
Silver nanostructures were created by the chemical reaction between copper film and silver nitrate (AgNO 3) as , followed by ultrasonication to obtain the colloidal solution. It was also concentrated by the same processes. Figure 4 shows SEM photos of (a) the silicon nanowire-modified surface, (b) the Au nanoparticle-modified surface, and (c) the Ag nanostructures on the electrode surface.
Figure 4 (a) SEM image of the silicon nanowire-modified surface, (b) SEM image of the Au-nanoparticle-modified surface, (c) Silver nanostructures on the electrode surface.
3. Experiments, results and discussions
3.1. Surface characterization
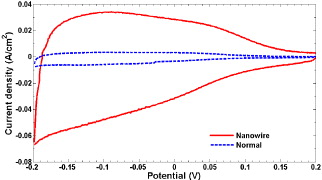
The surface area of the electrode was characterized quantitatively using cyclic voltammetry (CV). In this method, the roughness factor which is the ratio of the microscopic area (same as the active surface area) to the geometric area can be estimated with the graphical integral of its CV plot. This was discussed in detail in [2, 3]. Figure 5 shows the CV curves of the electrodes with and without silicon nanowire modification. The experiment was done in N 2-bubbled 1 M H 2 SO 4 solution at a scan rate of 100 mV s -1. The surface area increased 10.04 times [2] by comparing the graphical integrals. The CV characterization method could not be done to the Au and Ag nanostructure modified surfaces due to their surface properties of having more than one material. Therefore, we tested the signal magnitudes directly and used them to compare with the silicon nanowire modification.
Figure 5 Cyclic voltammogram of the electrodes with and without (labeled as 'normal') silicon nanowire modification.
3.2. Sensing neurotransmitters
The detection of neurotransmitters is based on chemical reactions generating H 2 O 2 which is then oxidized to release electrons. The electrons will form an electric current proportional to the concentration of the target neurotransmitter. Therefore, the probes could be calibrated with H 2 O 2 or an electro-active molecule that can directly release H 2 O 2 without any modification. For those which are not electro-active, a proper enzyme is needed to initiate the chemical reaction [2, 3]. We chose dopamine (DA) and L-glutamate. DA is electro-active, so it can be sensed directly using our electrodes. However, L-glutamate is not an electro-active molecule and requires the L-glutamate oxidase to initiate the following chemical reaction [2, 3]:
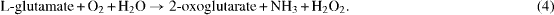
L-glutamate oxidase (USbio) was diluted in DI water and then mixed with bovine serum albumin (BSA) and glutaraldehyde (Sigma) to enhance the adhesion to surface [2, 3]. The enzyme mixture was then securely deposited to the working electrodes using a 10 μl Hamilton syringe. This step-by-step process was described in detail in [2, 3]. All the measurements were done with a 0.05 M phosphate-buffered saline (PBS) solution in a beaker under magnetic stirring. In experiments with L-glutamate sensors, the temperature of the PBS was controlled at 37 °C to have the best performance of the enzyme.
Among electrochemical sensing techniques, we chose amperometry due to its simplicity and accuracy. A fixed bias voltage of 0.7 V was applied between the working electrode and a commercial Ag/AgCl reference electrode placed together in the solution. Thirty minutes after immersing the electrodes in the solution, the signal would reach the baseline. Then desired chemicals were added in steps into the base solution and electrical currents were recorded continuously by a potentiostat.
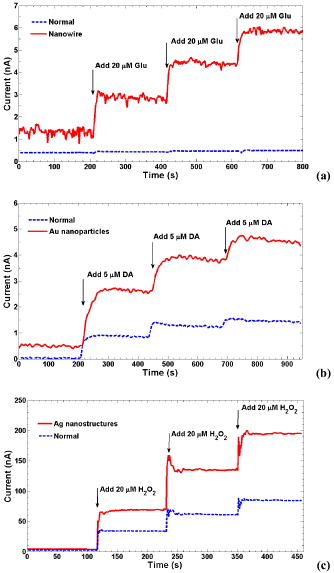
All modified devices were calibrated and tested with H 2 O 2, DA and L-glutamate, and some of the results are shown in figure 6. Figure 6(a) shows the enhancement of the nanowire-modified probe in detecting L-glutamate. Both the signal baseline and sensitivity were increased. The baseline rose from 0.4 to 1.3 nA while the slopes of responses, which represented sensitivity, were 0.0016 and 0.2044 nA μM -1for the sensors without and with nanowire modification, respectively. However, with greater levels in signals, higher-noise levels were also found since the increased active surface also increased the current contributed by noises. Therefore, the limit of detection (LOD) of these types of sensors should be investigated. The LODs were estimated as 4.8 and 1.7 μM for sensors without and with nanowire modification, respectively, based on the estimation of LOD as three times the baseline standard deviation [2]. The figure of merit was much lower than that of sensitivity; nevertheless, a higher signal level would not require a highly sensitive low-noise instrumentation amplifier, thus in return reducing the complexity, power consumption, size and costs of the recording electronics.
Figure 6 (a) Results of L-glutamate sensors with an electrode without modification (labeled as normal) and an electrode with silicon nanowire modified surfaces. (b) Responses to various DA concentrations in the normal and Au nanoparticles-modified electrodes. (c) Measurement results with various concentrations of H 2 O 2 on the Ag nanostructures' modified probe compared to the normal electrode surface.
Figure 6(b) shows the improvement of signal magnitudes in the Au nanoparticles' modified electrode compared to the one without modification for sensing DA. The baseline signal increased from 0.03 nA to 0.5 nA and an enhancement in sensitivity of 2.5 times from 0.16 to 0.4 nA μM -1 was obtained in the modified electrode. The LODs were estimated as 0.46 and 0.35 μM for sensors without and with modification, respectively,
Figure 6(c) shows the measurement result with H 2 O 2 for the silver nanostructure modified Pt electrode. The sensitivity increased from 1.9 to 3.7 nA μM -1 with surface modification. The LODs were estimated as 0.87 and 0.71 μM for sensors without and with modification, respectively.
The enhancement of output could be varied by using different densities of modification. With the VLS method, it could be done by varying the conditions of the PECVD processes, such as temperature, pressure and SiH4 flow rate; while in the Au and Ag cases, different concentrations of the colloid solutions could be used. The optimization of deposition density to reach optimal active surface areas will need further investigation.
4. Conclusion
We have developed micro-electrochemical probes aiming for neuroscience studies. The probes are based on rigid silicon and flexible polyimide substrates. Different nanostructure modification methods to enhance the performance of electrochemical neurotransmitter sensing were investigated. The silicon probes were modified with nanowires using the high-temperature VLS processes while the polyimide ones were modified by depositing nanostructures with colloidal solutions at room temperature. The modified silicon surfaces were characterized with SEM and cyclic voltammetry, and the nano-scale Au or Ag-modified surfaces were examined by SEM. Electrochemical experiments were realized to demonstrate the signal improvement. The signal levels and sensitivity were enhanced in H 2 O 2 calibration, DA and L-glutamate sensing experiments. Similar modification would be applied for other neurotransmitter sensors with proper chemical or biochemical functionalization on the nanostructured electrode surface.
Acknowledgments
The authors would like to thank Intel Corp. and Texas Norman Hackerman Advanced Research Program for their support.