Abstract
Hard magnetic strontium hexaferrite SrFe12O19 nanoparticles were synthesized by the sol–gel hydrothermal method. The factors affecting the synthesized process, such as the mole proportion of the reactants, pH, temperature, the hydrothermal conditions and the calcination process, have been investigated. The crystal structures of these materials were refined by Rietveld method. The obtained materials have single crystal phase, equal nano-size, plate shape and high anisotropy. The high magnetic coercivity of 6.3 kOe with the magnetization at 11.1 kOe of 66 emu g−1 at room temperature was observed for the strontium hexaferrite nanoparticles. For other nanoparticles (SrLnxFe12-xO19 and SrFe12O19/CoFe2O4) synthesized on the basis of SrFe12O19 the complex completion of the crystal structure distortion and the interaction between magnetic phases were observed.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Hard magnetic hexagonal ferrite materials MFe 12 O 19 (M=Ba, Sr) with magnetoplumbite structure have been widely applied in techniques to produce permanent magnets, loud speakers, microsized motors, actuators, micro wave devices, mini-pumps etc [1–3]. The advantages of these materials are the high coercivity, the moderate saturation magnetization and low price.
SrFe 12 O 19 has crystal structure of hexagonal M-type ferrite. The M-type ferrite crystallizes in a hexagonal structure with 64 ions per unit cell on 11 different symmetry sites. Each unit cell contains 10 closely packed oxygen layers forming SRS*R* hexagonal blocks. The 24 Fe 3+ atoms are distributed over five distinct sites: three octahedral sites (12k, 2a and 4f 2), one tetrahedral (4f 1) site and one bi-pyramidal site (2b). The Sr 2+ ions are located in the R blocks. The magnetic structure given by the Gorter model is ferrimagnetic with five different sublattices, three parallel (12k, 2a and 2b) and two anti-parallel (4f 1 and 4f 2) which are coupled by super exchange interactions through the O 2- ions [4]. The replacement of the Sr 2+ ions, which are non-magnetic and have large radius (1.18 Å) in crystal, by those with the same size and large charge such as La 3+ (1.032 Å), or by a magnetic ion such as Sm 3+ (0.958 Å), will induce deep changes of super exchange interactions between Fe 3+ ions through the O 2- ion at 2b site because the substitution of Sr 2+ by La 3+ or Sm 3+ reduces the oxidation number and distorts the bi-pyramidal sites containing Fe 3+.
The magnetic properties of this material are related to the fabrication method, particles size and particle morphology. So in the research of nano-SrFe 12 O 19 fabrication methods one should also care about high purity, ultrafine size, good dispersion and excellent magnetism [5–8]. The conventional method to create the SrFe 12 O 19 particles is solid phase reaction at very high temperature. Besides the solid route, chemical wet methods such as co-precipitation, hydrothermal, sol–gel etc, have been developed [5, 7–10]. The substitution of Sr 2+ in SrFe 12 O 19 crystal by rare earth ions (La 3+, Nd 3+, Sm 3+...) or the reaction between hard magnetic phase (SrFe 12 O 19) and a soft magnetic phase (CoFe 2 O 4, NiFe 2 O 4 ...) induces changes of magnetic properties of SrFe 12 O 19 [11–13].
In this paper, we present the sol–gel hydrothermal method for fabricating SrFe 12 O 19 nanoparticles and the relation between crystal structure and hard magnetic properties of SrFe 12 O 19 nanoparticles when the Sr 2+ ion was substituted by La 3+ and when the SrFe 12 O 19 is the core of the core–shell structure material. The sol–gel hydrothermal method combines the advantages of the sol–gel method and the high pressure in the hydrothermal condition. In the hydrothermal process, the particle size and particle morphology can be controlled. SrFe 12 O 19 nanoparticles have high purity, ultrafine size and high coercivity.
2. Experimental
2.1. Preparation of SrFe12 O19 nanoparticles
The samples were prepared using the sol–gel hydrothermal method. Chemical-grade Fe(NO 3)3·9H 2 O, Sr(NO 3)2 were used as pre-substances for preparing SrFe 12 O 19 (SFO) nanoparticles. A suitable amount of Fe(NO 3)3·9H 2 O and Sr(NO 3)2 was dissolved in distilled water to obtain Fe 3+ solution and Sr 2+ solution. These solutions were mixed in order to achieved the mole ratios of Fe 3+/ Sr 2+=8, 11, 12. Then citric acid (AC) solution was added to the mixture in the mole ratios AC/ΣMe=k (k=1, 2, 3; ΣMe is total mole of metal ions). The solution was stirred for about 1 h. The pH of the solution was adjusted to 6.5–7.5 with ammonium hydroxide, and subsequently the solution was heated until the volume decreased about 60%. Then sol continued to react in the autoclave at 180 °C. The achieved particles were dried and calcined at different temperatures in the air to form SrFe 12 O 19 nanoparticles.
2.2. Preparation of Sr(1-x) Lax Fe12 O19 nanoparticles
Sr (1-x) La x Fe 12 O 19 (x=0.05; 0.1; 0.2) nanoparticles were prepared by sol–gel hydrothermal method. Chemical-grade Fe(NO 3)3·9H 2 O, Sr(NO 3)2 and La 2 O 3 were used as pre-substances. A suitable amount of Fe(NO 3)3·9H 2 O and Sr(NO 3)2 was dissolved in distilled water to obtain Fe 3+ and Sr 2+ solution. A suitable amount of La 2 O 3 was dissolved in to HNO 3 solution to obtain La 3+ solution. These solutions were mixed in order to achieved the mole ratio of Fe 3+/(La 3++Sr 2+)=12. The next steps of the preparation process were the same as for the preparation of SrFe 12 O 19 nanoparticles.
2.3. Preparation of CoFe2 O4 nanoparticles
CoFe 2 O 4 nanoparticles were prepared by co-precipitation method. The mixed solution containing 50 ml of Fe 3+ and Co 2+ with the mole ratio Fe 3+/Co 2+=2 was drop-by-drop added into the reaction vessel containing 100 ml of 1 M NaOH aqueous solution under vigorous stirring. The suspension was stirred for 2 h. The precipitate was then separated by a high-speed centrifuge at 6000 rpm, and then washed with distilled water. The washed sample was dried at 100 °C in air for 2 h and then was sintered at different temperatures.
2.4. Preparation of SrFe12 O19/CoFe2 O4 core/shell structure
The right amount of SrFe 12 O 19 nanoparticles were dispersed in ethanol and sonicated for 10 min. Then NaOH solution of 1 M was used to activate the SrFe 12 O 19 nanoparticles by ultrasonic irradiation. After pre-treatment the SrFe 12 O 19 nanoparticles were separated by centrifugation. Then the SrFe 12 O 19 nanoparticles were added into the boiling NaOH aqueous solution. The mixed solution containing Fe 3+ and Co 2+ with the mole ratio Fe 3+/Co 2+=2 was drop-by-drop added into the reaction system and stirred for 2 h. The final product was separated by centrifugation, washed with distilled water, dried and sintered at different temperatures.
The magnetic properties of the products were observed using a vibrating sample magnetometer (VSM) at room temperature under a maximum field of 15 T. The reaction products were identified by x-ray diffraction (XRD) using Cu-Kα radiation and refined by Rietveld method using Rietan software. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to observe the grain size and morphology of the product.
3. Results and discussion
3.1. Characterization of SrFe12 O19
3.1.1. Influence of the mole ratio Fe3+/Sr2+
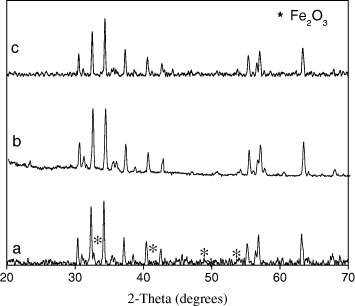
SrFe 12 O 19 nanoparticles were prepared with the mole ratio Fe 3+/Sr 2+=8, 11, 12. The XRD results showed that the samples with Fe 3+/Sr 2+=11 and 12 were single phase because we only observed the characteristic peaks of SrFe 12 O 19. The diffraction peaks at 2θ values of 34.171°, 32.311°, 30.341°, 37.151°, 63.161° and 56.81° corresponded to the strongest diffraction planes (114), (107), (110), (203), (2200) and (2011). These patterns ascribed to hexagonal Sr–M hexaferrite SrFe 12 O 19 phase. In the XRD patterns of sample with Fe 3+/Sr 2+=8, besides the peaks of SrFe 12 O 19, there were peaks of α-Fe 2 O 3. The peaks at 2θ values of 33.171°, 35.661°, 54.061°, 49.461° and 24.141° reflected the presence of Fe 2 O 3 phase. Figure 1 showed the XRD patterns of strontium M-ferrite powders with different Fe 3+/Sr 2+ mole ratio (8, 11 and 12) calcined at 1050 °C.
Figure 1 XRD patterns of SrFe 12 O 19 with different mole ratios of Fe 3+/Sr 2+ (a) SFO Fe 3+/Sr 2+=8; (b) SFO Fe 3+/Sr 2+=11 and (c) SFO Fe 3+/Sr 2+=12.
3.1.2. Influence of the mole ratio AC/ΣMe
SrFe 12 O 19 samples (the ratio of Fe 3+/Sr 2+=11 and 12) were prepared with different ratios of AC/ΣMe=k (k=1, 2 and 3).
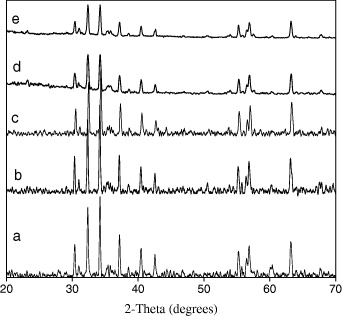
XRD patterns (figure 2) showed that all samples with AC/ΣMe=k=1, 2, 3 had high purity, SrFe 12 O 19 phase wasn't mixed with any different phase. So, the mole ratio AC/ΣMe did not affect the singles phase of SrFe 12 O 19 samples which were prepared by sol–gel hydrothermal method. However, the mole ratio AC/ΣMe affecting the magnetic properties of SrFe 12 O 19 samples and will be presented in VSM measurement results.
Figure 2 XRD patterns of SrFe 12 O 19 samples with different mole ratios of AC/ΣMe. (a) SFO12, k=1; (b) SFO12, k=2; (c) SFO12, k=3; (d) SFO11, k=2; (e) SFO11, k=3.
3.1.3. Influence of the sintering temperature
The sintering temperature was determined by the thermal analysis. The thermal analysis result of SrFe 12 O 19 (Fe 3+/Sr 2+=11, k=3) in the range 100–1200 °C is presented in figure 3.
Figure 3 Thermal analysis diagram of SrFe 12 O 19 sample with Fe 3+/Sr 2+=11, k=3.
The result shows that in the range of temperature of 200–500 °C the burning process of organic substances took place, at 500 °C, the burning process almost completed and total mass of sample reduced 72.45%. So the samples were preliminarily calcined at 500 °C. After the preliminary calcination, samples were sintered at temperatures of 550, 750 and 1050 °C. In the XRD patterns (figure 4) at 550 °C the only characteristic peak of α-Fe 2 O 3 was observed. At the higher temperature, 750 °C, SrFe 12 O 19 phase was formed, but a small amount of α-Fe 2 O 3 was still observed. At the sintering temperature of 1050 °C, single phase of SrFe 12 O 19 was formed. Figure 4 presents the XRD patterns of particle samples obtained after sintering at 550, 750 and 1050 °C.
Figure 4 XRD patterns of particle samples obtained after sintering at (a) 550, (b) 750 and (c) 1050 °C.
Figure 5 is the SEM micrograph of SrFe 12 O 19 phase (Fe 3+/Sr 2+=11, k=3, sintered at 1050 °C). One can observe that most of the particles are of hexagonal shape.
Figure 5 SEM micrograph of SrFe 12 O 19 phase (Fe 3+/Sr 2+=11, k=3, sintering at 1050 °C).
The average crystallite size was calculated by the Scherrer equation
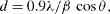
where d is average crystallite size; β—full width at half maximum (FWHM), λ=1.5406 Å—wavelength of X radiation. The average crystallite size of SrFe 12 O 19 is presented in table 1.
Table 1. Lattice parameters and average crystallite size of SrFe 12 O 19 samples.
| SFO11, k=2 | 5.87 | 5.87 | 23.00 | 686.88 | 7.13 | 9.47 | 7.4 |
| SFO11, k=3 | 5.87 | 5.87 | 23.01 | 687.38 | 8.05 | 10.95 | 7.8 |
In order to optimize the process of preparation of SrFe 12 O 19 nanoparticles and study the influences of preparation condition to material's magnetic properties, we varied the mole ratio Fe 3+/Sr 2+, the mole ratio AC/ΣMe and the calcination temperature.
The Rietveld method of structure refinement with powder diffraction patterns using Rietan software is used to refine the SrFe 12 O 19 structure. Calculated results showed that SrFe 12 O 19 with structure belongs to the P63/mmc space group, α=β=90 °, γ=120 ° and lattice parameters a, b, c and V are reported in table 1. The R p and R wp values under 10% show that the product prepared by sol–gel hydrothermal method has single phase and no other phase can be observed.
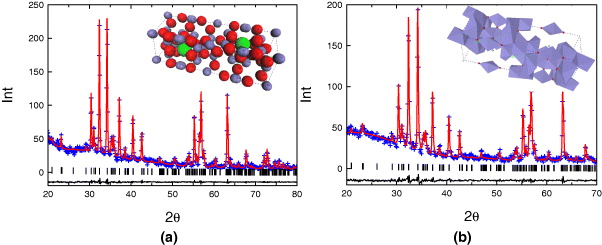
Figure 6(a) is the XRD result which was refined by Rietveld method and the simulated structure of SrFe 12 O 19 nanoparticles sintered at 1050 °C and kept for 2 h. The positions of atoms in the unit cell were calculated and are reported in table 2. The result of particles sample is close to the calculated result for bulk sample [14].
Figure 6 Simulated XRD pattern by the Rietveld method of SrFe 12 O 19 nanoparticles (a) and Sr 0.8 La 0.2 Fe 12 O 19 nanoparticles (b).
Table 2. The atomic positions in the unit cell of SrFe 12 O 19.
| Sr | 0.66667 | 0.33333 | 0.25000 | 0.605 |
| Fe(1) | 0 | 0 | 0 | −0.941 |
| Fe(2) | 0 | 0 | 0.25825 | −2.278 |
| Fe(3) | 0.33333 | 0.66667 | 0.02808 | −1.019 |
| Fe(4) | 0.33333 | 0.66667 | 0.18991 | −0.585 |
| Fe(5) | 0.16727 | 0.33824 | −0.10942 | −0.427 |
| O(1) | 0 | 0 | 0.15237 | −0.783 |
| O(2) | 0.33333 | 0.66670 | −0.05789 | 1.450 |
| O(3) | 0.16222 | 0.35362 | 0.25000 | −2.107 |
| O(4) | 0.15907 | 0.31305 | 0.05385 | 0.015 |
| O(5) | 0.47891 | 1.00758 | 0.14970 | −0.397 |
3.2. Characterization of Sr(1-x) Lax Fe12 O19
Because of the same ion radius between Sr 2+ and La 3+, when La 3+ is introduced into SrFe 12 O 19 system it tends to substitute the Sr 2+ position. Figure 6(b) is the XRD result which was refined by Rietveld method of the Sr 0.8 La 0.2 Fe 12 O 19 nanoparticles sintered at 1050 °C and kept for 2 h. No peak of any phases was observed except the characteristic peak of hexagonal structure of P63/mmc space group. The bond length of Fe 3+ ion and O 2− in the crystal does not change equally, but may increase or decrease depending on whether the tetrahedron or octahedron contains Fe 3+ ions distortion. However, the decrease of bond length of Fe 3+(2b)–O 2− (2b position is located in the same plane with M 2+ in crystal) can be observed when La 3+, whose ion radius is smaller than that of Sr 2+, substitutes the Sr 2+ position (table 3).
Table 3. The bond lengths between the atoms in the unit cell of Sr 0.8 La 0.2 Fe 12 O 19.
| Fe(1)–O(4) | 2.018 | 2.029 |
| Fe(2)–O(1) | 2.057 | 2.035 |
| Fe(2)–O(3) | 1.811 | 1.771 |
| Fe(3)–O(2) | 1.978 | 1.922 |
| Fe(3)–O(4) | 1.894 | 1.895 |
| Fe(4)–O(3) | 2.111 | 2.140 |
| Fe(4)–O(5) | 1.971 | 1.859 |
| Fe(5)–O(1) | 1.984 | 1.945 |
| Fe(5)–O(2) | 2.049 | 2.080 |
| Fe(5)-O(4) | 2.095 | 2.053 |
| Fe(5)–O(5) | 1.796 | 1.952 |
3.3. Characterization of SrFe12 O19/CoFe2 O4
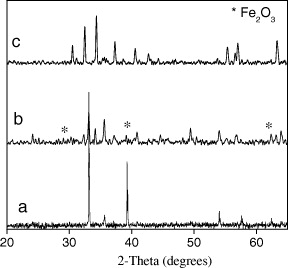
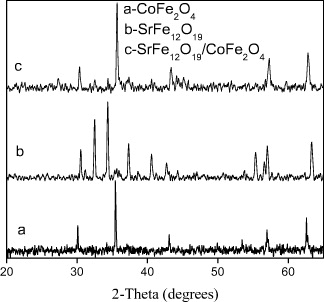
Figure 7 is the XRD results of the shell sample (CoFe 2 O 4), core sample (SrFe 12 O 19) and core/shell sample (SrFe 12 O 19/CoFe 2 O 4). On the XRD pattern of core/shell sample (SrFe 12 O 19/CoFe 2 O 4) the existence of two phase SrFe 12 O 19 and CoFe 2 O 4 was observed.
Figure 7 XRD patterns of core, shell and core/shell samples a-XRD pattern of CoFe 2 O 4 (shell); b-XRD pattern of SrFe 12 O 19 (core); c-XRD pattern of SrFe 12 O 19/CoFe 2 O 4 (core/shell).
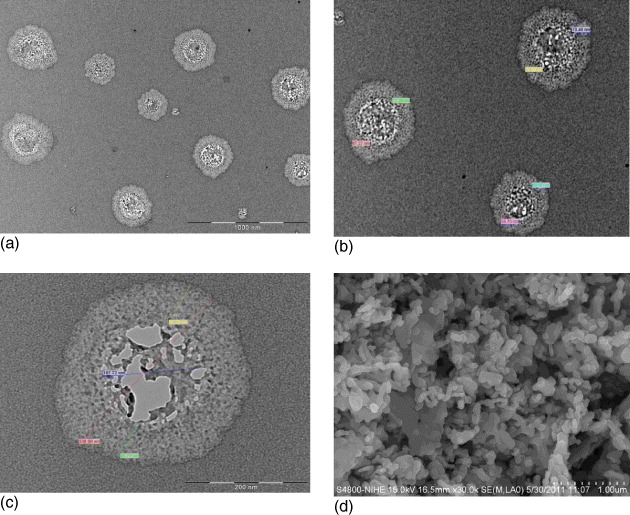
The core/shell structure of SrFe 12 O 19/CoFe 2 O 4 and the morphologies of hexaferrite phase and spinelferrite phase can be observed in the TEM and SEM photographs in figure 8.
Figure 8 TEM and SEM photographs of SrFe 12 O 19/CoFe 2 O 4 sample. (a, b, c) TEM images with different resolutions, (d) SEM image.
On the TEM of SrFe 12 O 19/CoFe 2 O 4 samples, one can observe the core–shell structure. The TEM results also show the dimension of the core–shell particles of SrFe 12 O 19/CoFe 2 O 4. The diameters of the core–shell particles are in the range of 265.86–354.22 nm, the diameters of the cores are in the range of 140.20–195.53 nm.
3.4. Magnetic properties
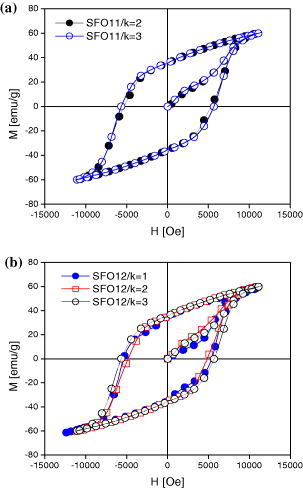
Figure 9 shows the VSM results at room temperature of SrFe 12 O 19 samples with Fe 3+/Sr 2+=11, 12; k=1, 2, 3 and sintered at 1050 °C. The magnetic properties of SrFe 12 O 19 samples are reported in table 4.
Figure 9 Hysteresis loops obtained by VSM of SrFe 12 O 19 samples with Fe 3+/Sr 2+ being 11 (a) and 12 (b); k=1, 2, 3 and sintered at 1050 °C.
Table 4. Magnetic properties of SrFe 12 O 19 samples.
| SFO11, k=2 | 60 | 35 | 5420 |
| SFO11, k=3 | 66 | 38 | 6315 |
| SFO12, k=1 | 59 | 35 | 5470 |
| SFO12, k=2 | 60 | 35 | 4911 |
| SFO12, k=3 | 60 | 35 | 5665 |
| Sr 0.8 La 0.2 Fe 12 O 19 | 63 | 37 | 5955 |
Saturation magnetization of SFO samples with different mole ratios Fe 3+/Sr 2+ and Ac/ΣMe are not very different, but the SFO sample with the mole ratio Fe 3+/Sr 2+=11 and k=3 has the highest saturation magnetization (Ms=66 emu g −1). This sample also has the highest coercivity (H c =6315 Oe). Other samples have high coercivity in the range of 4911–5665 Oe. The coercivity of SrFe 12 O 19 depends on the anisotropy of the grain, and coercivity increases with the increase of anisotropy. It can be observed that the higher the mole ratio AC/ΣMe=k, the higher are the coercivity values.
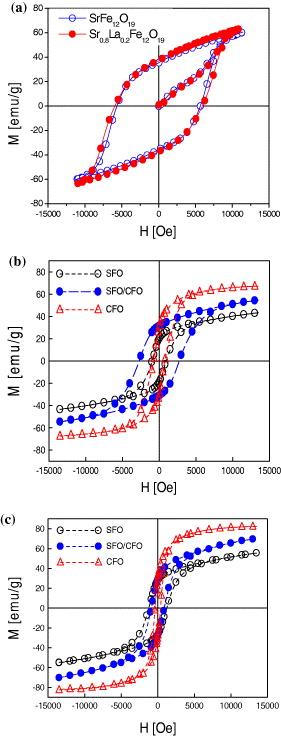
Figure 10(a) is the hysteresis loops of SrFe 12 O 19 and Sr 0.8 La 0.2 Fe 12 O 19 nanoparticles prepared by hydrothermal method in the same conditions. The substitution of Sr 2+ by La 3+ created the change of coercivity. H c value of Sr 0.8 La 0.2 Fe 12 O 19 is higher than that of SrFe 12 O 19. Figures 10(b) and (c) are the hysteresis loops of the SrFe 12 O 19/CoFe 2 O 4 nanoparticles after calcination at 950 °C (figure 10(b)) and 1050 °C (figure 10(c)) for 2 h.
Figure 10 Room temperature hysteresis loops (a) SrFe 12 O 19 and Sr 0.8 La 0.2 Fe 12 O 19 1050 °C, (b) SrFe 12 O 19/CoFe 2 O 4950 °C and (c) SrFe 12 O 19/CoFe 2 O 41050 °C.
4. Conclusion
The SrFe 12 O 19 and Sr 1-x La x Fe 12 O 19 nanoparticles were synthesized successfully by sol–gel hydrothermal method. The obtained products have single phase, particles have hexagonal shape, the right proportion and high coercivity. The Rietveld method of structure refinement with powder diffraction patterns using Rietan software is used to determine the SrFe 12 O 19 and Sr 1-x La x Fe 12 O 19 structure. The calculated results agree with experiment.
The SrFe 12 O 19 synthesis process by sol–gel hydrothermal method was optimized, SrFe 12 O 19 nanoparticles have high purity, ultrafine size and high coercivity (H c =4911–6315 Oe). Coercivity values depend on the mole ratios Fe 3+/Sr 2+ and AC/ΣMe, samples with Fe 3+/Sr 2+=11 and k=3 has the highest coercivity (H c =6315 Oe). In the same synthesis conditions of mole ratio AC/ΣMe, pH, the sintering temperature, the coercivity of SrFe 12 O 19 is higher than that of Sr 1-x La x Fe 12 O 19.
The physical parameters of nanoparticles with core-shell structure SrFe 12 O 19/CoFe 2 O 4 were calculated. The diameters of the core-shell particles are in the range of 265.86 nm–354.22 nm, the diameters of the cores are in the range of 140.20–195.53 nm. The core-shell structure was observed by TEM photographs.
The substitution of Sr 2+ position by La 3+, the interaction between hard and soft magnetic phases and the synthesis method strongly affected the structure and magnetic properties of SrFe 12 O 19 nanoparticles.
Acknowledgments
This work received support from NAFOSTED (104.02.74.09) and MOET (B2010-01–321).