Abstract
Gold, silver and copper nanoparticles were prepared in clean and biologically-friendly liquids by laser ablation. The average size of nanoparticles ranges from 3 to 30 nm. These nanoparticles were used to fabricate nanostructured substrates for surface enhanced Raman scattering (SERS) measurement. Raman spectra were measured by a Micro-Raman spectrophotometer. The results show that gold, silver and copper nanoparticle substrates fabricated by our method are effective for SERS studies. SERS was also obtained when using gold, silver and copper nanoparticle colloid prepared by laser ablation.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) was originally discovered in the 1970s. Recently, almost 30 years after the discovery of SERS, interest in SERS has exploded. Thanks to exciting advances in techniques for preparing nanoparticles and to the laser and optics technology associated with measuring the Raman spectra, a number of important applications have been reported [1–3].
Under favorable circumstances, Raman enhancements as large as 14 orders of magnitude can be achieved. This is a very high degree of enhancement, capable of single-molecule detection. It raises a great interest in creating an ultrasensitive sensing platform based on SERS with molecular identification capabilities, especially as a sensor for biological molecules. SERS allows the detection of organic molecules adsorbed on metal substrates (such as gold, silver, copper etc) at submicromolar concentration.
The large enhancement of the Raman scattering intensity has been explained by two mechanisms: the electromagnetic and chemical mechanisms [1, 2]. The electromagnetic mechanism is attributed to the increase of the local electromagnetic field of the adsorbate because of the excitation of the surface plasmon on the metal surface. The chemical adsorption mechanism is attributed to short distance effects due to the charge transfer between the metal and the adsorbed molecule [3].
The electromagnetic effect is dominant and the chemical effect contributes to the enhancement only on one or two orders of magnitude [3]. The electromagnetic enhancement (EME) is dependent on the presence of the metal surface's roughness features, while the chemical enhancement (CE) involves changes to the adsorbate electronic states due to chemisorption of the analyte [3, 4].
Surface roughness or curvature is required for the excitation of surface plasmon by light. The electromagnetic field of the light at the surface can be greatly enhanced under conditions of surface plasmon excitation; the amplification of both the incident laser field and the scattered Raman field through their interaction with the surface constitutes the electromagnetic SERS mechanism.
It follows from the experiments and theoretical models [4] that the maximum SERS enhancements are observed when λ sp =(λ exc +λ Rs )/2, where λ sp , λ exc and λ Rs are the surface plasmon resonance, excitation and Raman wavelengths, respectively. Differences between various metals (silver, gold, copper ...) can lead to differences in the SERS enhancement. This is explained by the electromagnetic model simultaneously enunciated in 1980 by Gersten [5], Gersten and Nitzan [6] and McCall and Platzman [7]. According to this model, the polarizability of a small metal sphere with dielectric function ε (λ) and radius R surrounded by vacuum is given by:
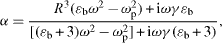
where ε b is the (generally wavelength-dependent) contribution of interband transitions to the dielectric function, ω p is the metal's plasmon resonance frequency whose square is proportional to the electron density in the metal and γ is the electron-scattering rate that is inversely proportional to the electronic mean-free-path and therefore also inversely proportional to the metal's dc conductivity. The real and imaginary parts of the expression for α have a pole when the frequency ω is equal to . The width of that resonance is given by γ (ε b +3) [2].
Hence, when γ is large either due to the inherent poor conductivity of the metal or because the metal nanofeatures are so small that electronic scattering at the particle's surfaces become the dominant interaction process, the quality of the resonance and SERS enhancement is reduced. Likewise, for metals whose dielectric properties are greatly modified by interband transitions in the wavelength range under consideration, i.e. for which the value of the function ε b is large, the width of the resonance increases and the SERS-enhancement decreases. Most transition metals are poor SERS enhancing systems because, for them, the two effects combine to reducing their SERS enhancement ability, i.e. their conductivity is low (γ is large) and the interband contribution to the dielectric function is great (ε b is large) [2].
In this paper we used noble metal nanoparticles for SERS substrate. The gold, silver and copper nanoparticles were prepared by laser ablation in clean liquid environment without contamination. It is well known that the nanoshell's plasmon resonance in the SERS substrate generates a strong signal. We study the fabrication of an efficient SERS substrate containing noble metal nanoparticles.
2. Experimental
Gold, silver and copper nanoparticles were prepared by laser ablation of corresponding noble metal plates in ethanol. The noble metal plate (99.9% in purity) was placed in a glass cuvette filled with 10 ml ethanol. The fundamental wavelength (1064 nm) of the Quanta Ray Pro 230 Nd:YAG laser was focused on the metal plate by a lens having the focal length of 150 mm. A small amount of the metal nanoparticles colloids was extracted for absorption measurement and transmission electron microscopy (TEM) observation. The absorption spectrum was measured by a Shimadzu UV-2450 spectrometer. The TEM micrograph was taken by a JEM 1010-JEOL. The size of nanoparticles was determined by ImageJ 1.37v software of Wayne Rasband (National Institutes of Health, USA). The size distribution was obtained by measuring the diameter of more than 500 particles and using Origin 7.5 software.
SERS substrate was prepared by dropping metal nanoparticle colloids on a silicon wafer or glass slide. A rohdamine 6G (R6G) solution of 10−4 M concentration in ethanol was used as a test analyte to study SERS spectrum. A few droplets of the analyte solution were dropped and left to be dried on the SERS substrate. R6G molecules were absorbed onto the metal nanoparticles of the SERS substrate after some minutes. SERS spectra were observed by micro-Raman spectrophotometer (Micro Raman LabRAM - 1B and LabRAM HR).
3. Results and discussion
3.1. Preparation of gold, silver and copper nanoparticles in ethanol
We chose ethanol as surfactant for metal nanoparticles because ethanol can evaporate quickly in the preparation of SERS substrate by our method. The morphology and size of metal nanoparticles depend on many factors in laser ablation process such as laser fluency, laser irradiation, laser wavelength and concentration of surfactant solution. We controlled all these factors to get a suitable laser ablation procedure. Using 1064 nm wavelength of Nd:YAG laser with average power of 400 mW, irradiation time of 15 min we prepared gold, silver and copper nanoparticles in ethanol. The results were presented in figure 1.
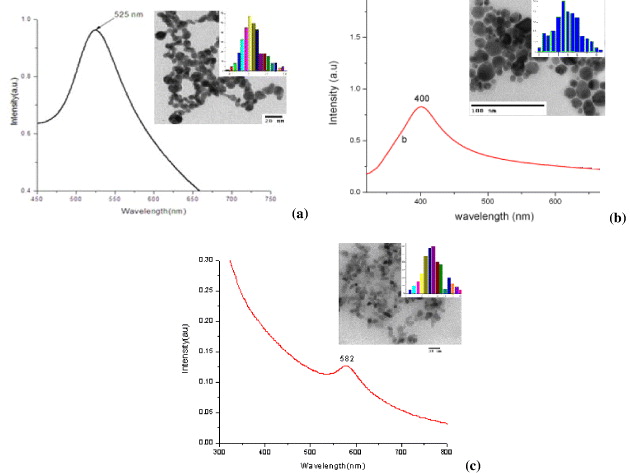
Figure 1 Absorption spectra ,TEM images and size distribution of gold (a), silver (b) and copper (c) nanoparticles prepared by laser ablation in ethanol.
The characteristic plasmon resonance absorption peaks around 520, 400 and 580 nm of the gold, silver and copper nanoparticles, respectively, were observed. The TEM images of gold, silver and copper nanoparticles show that the metal nanoparticles are rather spherical in shape. The data of size and size distribution of metal nanoparticles were analyzed and given in the inset. Analysis from size distribution shows that the mean diameter of gold, silver and copper nanoparticles is 13, 15 and 12 nm, respectively.
3.2. Raman spectra of R6G on SERS substrates
A layer of gold nanoparticles was prepared on silicon wafer to fabricate a SERS substrate. In order to examine the Raman enhance effect of the SERS substrates we prepared three types of different samples: R6G on silica substrate without Au nanoparticle colloid (R6G/Si sample), Au nanoparticle colloid on silica substrate without R6G (Au/Si sample), R6G on silica substrate with Au nanoparticle colloid (R6G/Au/Si sample). The spectra measured by micro-Raman spectrophotometer of these three samples are shown in figure 2.
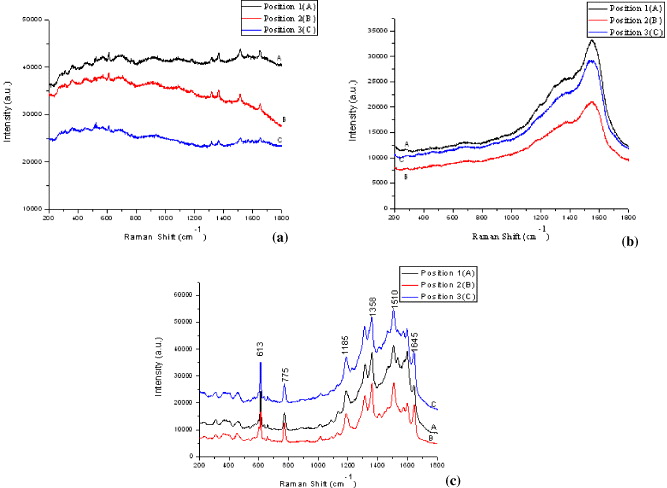
Figure 2 Raman spectra of R6G/Si sample (a), Au/Si sample (b) and R6G/Au/Si sample (c) taken in three different positions of samples.
The spectra are quite similar for three random different positions of the samples. Comparing Raman spectra of R6G/Si and R6G/Au/Si samples we can conclude that Raman signal was enhanced strongly by SERS substrate prepared by our procedure.
The SERS spectra of R6G are in good agreement with the published results of R6G Raman spectrum. Strong peaks at 1358, 1510 and 1645 cm −1 are assigned to the C–C stretch. The peak at 613 cm −1 assigned to the C–C–C deformation in-plane vibration was experimentally observed at 612 cm −1. It is explained by the plasmon-generated electric field [8–10]. The peak at 775 cm −1 is to assign the C–H deformation band out-of-plane vibrations. The peak at 1185 cm −1 indicates the C–H deformation in-plane vibration. However, these wavenumbers are slightly different from those in the normal Raman spectrum of R6G. It indicates that there is a slight change in the secondary structure of R6G because of the interactions between R6G molecules and gold nanoparticles [8].
By the same method we prepared SERS substrates by silver and copper nanoparticles and observed Raman spectra of the samples. The SERS spectra of R6G adsorbed on silver and copper nanoparticle substrates are shown in figure 3.
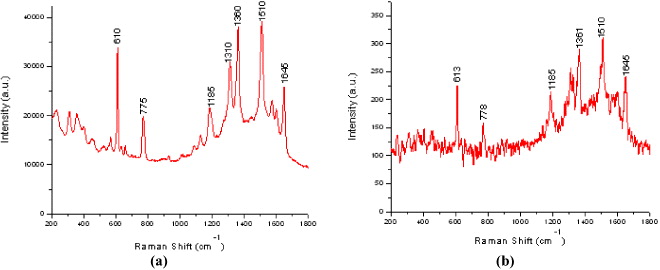
Figure 3 SERS spectra of R6G absorbed on Ag (a) and Cu (b) nanoparticle substrates.
There are differences of peak intensities in SERS spectra obtained from R6G/Au/Si sample, R6G/Ag/Si sample and R6G/Cu/Si samples. The Raman enhancement effect of silver nanoparticles is higher than those of gold and copper nanoparticles. SERS effect of Cu nanoparticles is lowest. This can be explained by the parameter ε b (the contribution of interband transition to the dielectric function). When ε b is large, the width of the resonance increases and the SERS enhancement decreases [2, 8]. The contribution of interband transitions in the dielectric function of those metals in the visible range of the spectrum increases in that order.
3.3. Raman spectrum of R6G in gold nanoparticle colloid
In order to examine the SERS enhancement effect of metal nanoparticle colloids we observed Raman spectrum of R6G in gold nanoparticle colloid. The result is shown in figure 4.
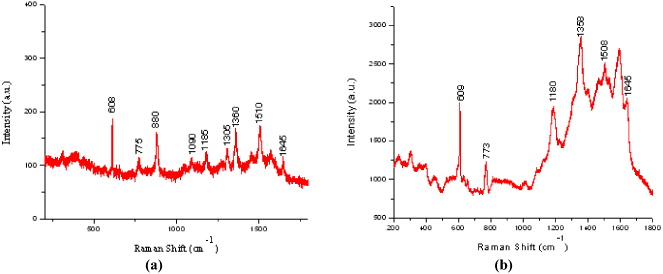
Figure 4 SERS spectra of R6G in ethanol (a) and in Au nanoparticle colloid (b).
The characteristic Raman peaks (608, 773, 1080, 1510, 1645 cm −1 ...) of R6G solution 10−5 M in ethanol are very weak in comparison with Raman peaks of ethanol (880, 1050, 1096 cm −1) (figure 4(a)). The intensity of R6G Raman peaks becomes much stronger in the presence of Au nanoparticles in ethanol (figure 4(b)). These results indicated the possibility of our method for quantitative SERS spectrum detection.
4. Conclusion
Gold, silver and copper nanoparticle colloid prepared by laser ablation in ethanol was used to fabricate SERS substrates. The Raman signal is strongly enhanced by our SERS substrate. The Raman enhancement effect of silver nanoparticles is higher than those of gold and copper nanoparticles. The experimental results showed advantages of the laser ablation method for preparing metal nanoparticles in the clean liquids suitable for SERS studies.
Acknowledgments
This research was supported by the project 43/2009/HD-NDT Vietnam-Russia and the project QGTD-10.04 of VNU HN, Vietnam.