Abstract
Zinc oxide nanoparticles (ZnO NPs) have attracted great attention because of their superior optical properties and wide application in biomedical science. However, little is known about the anticandidal activity of ZnO NPs against Candida albicans (C. albicans). This study was designed to develop the green approach to synthesize ZnO NPs using egg white (denoted as EtZnO NPs) and investigated its possible mechanism of antimicrobial activity against C. albicans 077. It was also notable that anticandidal activity of EtZnO NPs is correlated with reactive oxygen species (ROS) production in a dose dependent manner. Protection of histidine against ROS clearly suggests the implication of ROS in anticandidal activity of EtZnO NPs. This green approach based on egg white-mediated synthesis of ZnO NPs paves the way for developing cost effective, eco-friendly and promising antimicrobial nanomaterial for applications in medicine.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The brutality of antifungal drugs in pharmacotherapy has led to the development of widespread multidrug resistance (MDR) in Candida albicans [1, 2]. Unfortunately, most of these drugs, being chemical in nature and having bulk form, are too reactive and unsuitable for human use [3]. With the rising toxicity and development of MDR in C. albicans isolates, the search for new medical treatments beyond conventional antifungal drugs has become a key aim of public health research [4, 5]. The failure of drugs to control infection makes it crucial to find new alternatives to currently available drugs. Possible innovative strategies encompass the inhibition of C. albicans growth through the use of nanomaterials.
Zinc oxide (ZnO) nanoparticles (NPs) are being widely used in health care commercial products due to their unique properties such as UV light absorption, and being catalytic, semi-conducting, magnetic and antimicrobial [6–8]. ZnO NPs exhibit unique characteristics that may completely differ from bulk-sized ZnO materials in terms of higher proportion of atoms on the surface of nano-sized materials, light absorption, electronic band gap, and being catalytic and antimicrobial compared to bulk-sized ones [7–11]. Therefore, owing to the large surface-to-volume ratio, ZnO NPs give rise to high reactivity towards biological responses [12, 13]. Numerous studies suggest that ZnO particles with different sizes and shapes have different degrees of antimicrobial activities [12, 14–17]. ZnO NPs also have excellent stability, robustness, biocompatibility and longer shelf life compared to other antimicrobial agents [12, 18]. Although the toxic impact of ZnO NPs on living cells has been proved, relatively low concentrations of ZnO NPs are non-toxic to eukaryotic cells [18–22]. Consequently, ZnO NPs are believed to be non-toxic, biosafe and have been used as drug carriers cosmetics and fillings in medical materials [13, 18, 23, 24].
Multiple lines of evidence propose that ZnO NPs increase oxidative stress through excess production of reactive oxygen species (ROS), namely hydroxyl radicals (•OH) and singlet oxygen (1O2), which dominantly contribute to the antimicrobial activity of ZnO NPs [12, 19, 25–28]. Other mechanisms such as cytoplasmic membrane disruption and electrostatic binding of ZnO NPs to the cell surface of the microbial pathogen have been reported [19, 27]. Although many controversies have been raised on the mechanistic aspects of the antimicrobial activity of ZnO NPs, recently the production of ROS governed by the electronic band gap property of metal-oxide NPs were considered to trigger the actual 'mechanism' [28]. The electronic band gap property of ZnO NPs is influenced by the structural parameters (size and pH) and carrier molecule concentrations [29, 30]. The debate in the scientific community continues in determining exact mechanisms that give rise to the antimicrobial properties of ZnO NPs. Therefore, a fundamental understanding on electronic band gap property of ZnO NPs becomes crucial to the tailoring of the new antimicrobial metal oxide NPs and effectively reduces experimental testing cost.
The properties of ZnO NPs are strongly dependent on their morphology and the size of the crystal [31]. Morphology and size can play a key role in many optoelectronic and antimicrobial applications. Therefore developing a shape-controlled ZnO NPs synthesis method is crucial for exploring the potential of ZnO NPs as a source of smart and functional materials. To date, numerous distinct molecules such as tri-n-octylphosphine oxide (TOPO), sodium dodecyl sulfate (SDS), polyoxyethylene stearyl ether (Brij-76), bovine serum albumin (BSA) and citric acid have been used to control the size and shape of ZnO NPs during synthesis [11, 32]. In recent years, researchers have begun to use natural bioresource with excellent biocompatibility as a template for the synthesis of nanomaterials. However, information about mechanisms of antimicrobial potential of ZnO NPs synthesized via egg albumen acting as a template is not available. There is thus a need to identify a novel class of biocompatible anticandidal ZnO NPs, which could present us with new opportunities for the development of safe and effective antibiotic drugs for treating C. albicans [33, 34]. In present investigation we synthesized anticandidal ZnO NPs using egg albumen as a biotemplate.
2. Experimental details
2.1. Materials
Zinc acetate, potassium bromide, ammonia and other chemicals were procured from SRL, India. Sabouraud's dextrose (SD) nutrient media for the cultivation of C. albicans 077 were obtained from the HiMedia Laboratories, Mumbai, India. 2,7-dichlorofluorescin diacetate (DCFH-DA) and histidine were obtained from Sigma Aldrich (St Louis, Missouri, USA). All other chemicals used were of the highest purity available from commercial sources.
2.2. Synthesis of ZnO NPs
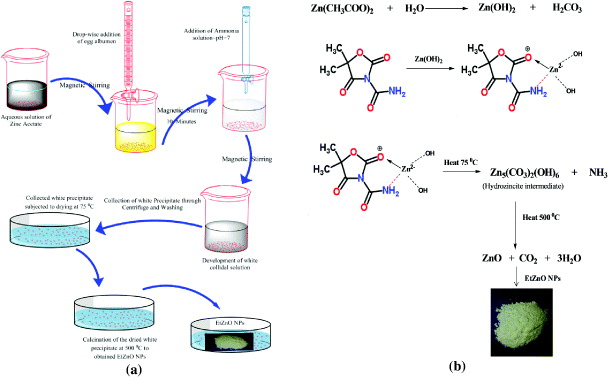
The synthesis of ZnO NPs using egg albumin as a biotemplate (denoted as EtZnO NPs) was performed according to the method of Nouroozi and Farzaneh [35]. In brief, freshly extracted 20 ml of egg albumens (5 mg ml−1) were prepared in mixed milli-Q (MQ) water and mixed drop-wise into 80 ml of aqueous zinc acetate [Zn(CH3COOH)2ċ2H2O] solution. The mixture was stirred for 10 min at room temperature to form the colloidal solution. The colloidal solution was precipitated by the addition of ammonia (NH3) at ∼pH 7.0 and centrifuged at 5000 rpm for 10 min and twice washed with the MQ water. The washed material was collected and dried in the vacuum oven and grounded into a fine powder (figure 1(a)). The obtained dried powder was subjected to sintering at 500 °C for 3 h and synthesized EtZnO NPs were stored in a dry and dark place until further use (figure 1(b)).
Figure 1. Systematic representation of synthesis of EtZnO NPs. (a) Synthesis method of EtZnO NPs and (b) mechanistic aspect of the synthesis of EtZnO NPs using egg albumen as a biotemplate.
Download figure:
Standard image High-resolution image2.3. Characterization of EtZnO NPs
The x-ray diffraction (XRD) patterns of the powdered sample were recorded on a MiniFlex™ II benchtop XRD system (Rigaku Corporation, Tokyo, Japan) operating at 40 kV [36]. For the morphological analysis, transmission electron microscopy (TEM) of aqueous EtZnO NPs was carried out on JEOL 100/120 kV TEM (JEOL, Tokyo, Japan) with an accelerating voltage of 80 kV. Briefly, a drop of aqueous EtZnO NPs was placed on the carbon coated copper grid and air dried under dark. The elemental analysis was determined using the Oxford Instruments INCAx-sight energy dispersive x-ray (EDAX) spectrometer equipped TEM. Thin film of the EtZnO NPs was prepared on the borosilicate glass slide for the analysis of surface morphology. The prepared thin film was analyzed on the atomic force microscope (AFM) Innova SPM Veeco in tapping mode. Commercial etched silicon tips as scanning probes with typical resonance frequency of 300 Hz (RTESP, Veeco) were used. The microscope was placed on a pneumatic anti-vibration desk under a damping cover and analysis was performed using SPM Lab software. The electron, EDAX and AFM images were obtained and converted into an enhanced meta file format. For the Fourier transform infrared (FTIR) spectroscopic measurements, the EtZnO NPs powder was mixed with spectroscopic grade potassium bromide (KBr) in the ratio of 1:100 and spectra were recorded in the range of wavenumber of 400–4000 cm−1 on Perkin Elmer FTIR Spectrum BX (PerkinElmer Life and Analytical Sciences, CT, USA) in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets.
The synthesis of EtZnO NPs in the solution was monitored by recoding the absorbance (A) using UV–Vis spectrophotometer (PerkinElmer Life and Analytical Sciences, CT, USA) in the wavelength range of A200 to A800nm. Fluorescence spectra were obtained on a spectrofluorometer RF 540 (Shimadzu, Japan) with the sample placed in 1 cm path quartz cuvette with excitation wavelength ∼370 nm. The thermal stability of the EtZnO NPs was investigated by thermogravimetric analysis (TGA) (Sieco SII, SSC5100, Instrument) at a heating rate of 10 °C min−1 under nitrogen atmosphere. The dispersal of EtZnO NPs in SD broth medium and MQ water were stored for 40 h and 90 days, respectively, and the ∼A370nm were measured regularly to ascertain their stability.
2.4. Assessment of anticandidal activity of EtZnO NPs
2.4.1. Growth condition.
Clinical isolate of C. albicans 077 was obtained from Department of Microbiology, JN Medical College, Aligarh, UP, India. Stock culture was maintained on slants of SD agar (containing dextrose 40 g l−1, mycological, peptone 10 g l−1 and agar 15 g l−1) at 4 °C. The primary culture of the C. albicans 077 was prepared from the stock slant into the SD broth medium and incubated at 37 °C for 48 h (stationary phase, 108 cfu ml−1). The primary culture (∼1 ml) was re-inoculated into the 50 ml fresh SD broth and grown for ∼12 h up to mid-log phase (∼105 cfu ml−1) at 37 °C. All experiments were performed from the mid-log phase (∼105 cfu ml−1) freshly grown C. albicans 077 culture in triplicates.
2.4.2. Agar disc diffusion assay.
For agar disc diffusion assay, 5 ml mid-log phase grown of C. albicans 077 was centrifuged at 4000 rpm for 5 min at 4 °C. Then, the pellet was washed with 1 × phosphate buffer saline (PBS) and resuspended in 500 μl normal saline solution (NSS). A 100 ml of the suspended cells were spread uniformly on SD agar plates and the plates were incubated at 37 °C for 30 min. The seeded petri plates were used for loading various concentrations of EtZnO NPs (0, 5, 10 and 15 μg ml−1) onto the pre-sterilized filter paper discs. The petri plates were incubated at 37 °C for 40 h, after that zone of inhibition was recorded.
2.4.3. In vitro killing assay.
Cell suspension of C. albicans 077 was obtained similarly as described in the agar disc diffusion assay and suspension (100 μl) was dispensed into the 96-well microtiter plate in triplicates. Various concentrations of EtZnO NPs (0, 5, 10 and 15 μg ml−1) diluted in sterile SD broth medium were added and incubated at 37 °C for 2 h. The whole suspension of the plate wells was spread on the SD agar plate and incubated at 37 °C for 40 h. Anticandidal activity was detected by the dose dependent reduction in cfu ml−1.
2.4.4. Growth kinetics assay.
To see the effect of EtZnO NPs on the growth kinetics of C. albicans, 50 ml of SD broths in individual flask were inoculated with 100 μl of the NSS suspended cells. Different concentrations of EtZnO NPs (0, 5, 10 and 15 μg ml−1) to be tested were applied in the individual flask. The flasks were incubated at 37 °C for 40 h and time dependent growth kinetics were recorded turbidometrically at A595nm. The turbidity backgrounds induced by EtZnO NPs were subtracted from the final reading.
2.5. Measurement of intracellular ROS generation
The produced intracellular ROS was measured using 2,7dichlorofluorescin diacetate (DCFH-DA) [37]. The DCFH-DA passively enters the cell where it reacts with ROS to form highly fluorescent dichlorofluorescein (DCF). Briefly, DCFH-DA (10 mM) stock solution in methanol (HPLC grade) was diluted in culture medium to yield a working solution (100 μM). At the end of exposure, C. albicans 077 cells were washed twice with ice-cold 1 × PBS and then incubated in 1 ml of working solution of DCFH-DA at 37 °C for 30 min. The C. albicans 077 cells were treated with different concentrations of EtZnO NPs for 40 h, lysed in alkaline solution and centrifuged at 5000 rpm for 10 min. Then, a 200 μl of supernatant was transferred to the other fresh well of microtiter plate and fluorescence was measured at excitation of A485nm and emission of A520nm using a microplate reader (Bio-Rad laboratories Inc., Hercules, CA, USA).
3. Results and discussion
3.1. Characterization of EtZnO NPs
3.1.1. Structural characterization.
The crystal structure of EtZnO NPs was characterized by XRD with CuKα radiation (λ = 0.15418 nm). The data revealed that the well resolved 11 XRD peaks were obtained at 2θ = 31.01°, 34.21°, 35.64°, 47.10°, 56.02°, 62.15°, 65.68°, 67.51°, 69.01°, 72.08° and 76.24° which correspond to the crystal planes [100], [002], [100], [102], [110], [103], [200], [112], [201], [004] and [202] of polycrystalline wurtzite structure (Zincite, JCPDS 5-0664), respectively (figure 2(a)). The XRD data of EtZnO NPs indicated the absence of any impurities, which attested its high quality. The average crystallite size (D) of EtZnO NPs was calculated following the Debye–Scherrer formula
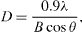
where constant 0.9 is the shape factor, λ is the x-ray wavelength of CuKα radiation (1.54 Å), θ is the Bragg diffraction angle and β is the full-width at half-maximum (FWHM) of the (101) plane diffraction peak. The calculated average particle size was found to be ∼16 nm.
Figure 2. EtZnO NPs structural characterizations. (a) XRD patterns of EtZnO NPs were recorded in the range of 20–80° of 2 θ angles. XRD pattern of EtZnO NPs depicted the well-resolved diffraction peaks of the crystalline wurtzite particles structure. (b) SEM of EtZnO depicts the microstructure EtZnO NPs. (c) The EDAX spectrum proves the elemental composition of as-synthesized EtZnO NPs. (d) EDAX map indicates the presence of Zn and O. (e) TEM of EtZnO depicts the NPs structure. (f) 2D and (g) 3D atomic force micrographs illustrate the nanostructure of as- synthesized EtZnO NPs, respectively.
Download figure:
Standard image High-resolution imageThe nanostructure of synthesized EtZnO NPs was analyzed by SEM, TEM and AFM. The figure 2(b) showing the scanning electron micrograph recorded from EtZnO NPs film deposited on a carbon tape clearly demonstrates the formation of secondary EtZnO NPs (average size ∼1 μm). The elemental analysis using EDAX indicates the presence of only zinc and oxygen in as-synthesized EtZnO NPs (figure 2(c)). Moreover, the EDAX mapping result again shows that there are no other elemental impurities present in the synthesized EtZnO NPs (figure 2(d)). The TEM image clearly showed the EtZnO NPs to be actually composed of several particles of different sizes grouped in clusters (figure 2(e)). It can also be seen that EtZnO NPs have a spherical morphology with a size range of 10–20 nm, which is consistent with XRD and optical results [38].
TEM characterization of EtZnO NPs revealed that all NPs were stable, well dispersed, smooth and the agglomeration might be due to the preparation technique. The particles were deposited on a copper grid and drying promotes agglomeration [37, 39]. The AFM images also display the spherical morphology of EtZnO NPs in the size range of ∼8–22 nm (figures 2(f) and (g)), which is consistent with results obtained by the XRD and TEM. Thus, the data confirmed that the EtZnO NPs were successfully synthesized using the egg albumen as a biotemplate.
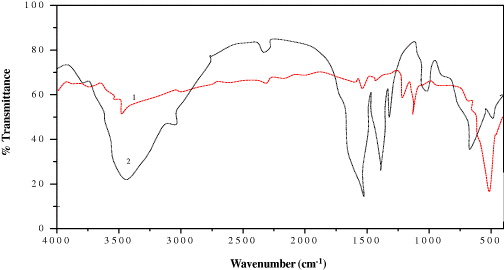
The comparative analysis of the FTIR spectra revealed possible interactions between egg albumen and ZnO NPs. The FTIR spectrum of EtZnO NPs shows absorption band at 511 cm−1, which corresponds to E2 mode of hexagonal ZnO wurtzite structure [40]. The band at the position 511 cm−1 reflects EtZnO NPs stretching frequency of Zn–O bonds. The intermediate product hydrozincite (without sintering) shows the absorption band at 677 cm−1 due to the presence of the hydroxide phase of the EtZnO NPs (figure 3). The sintering process also indicated the disappearance of the egg albumen signature peaks which confirmed its decomposition from the EtZnO NPs. The comparative FTIR spectra confirmed that the transformation of the hydroxide to the oxide phase occurred during the sintering process of EtZnO NPs. The bands at ∼1557 and 3452 cm−1 were due to the stretching frequency of hydroxyl groups of absorbed water from ambient atmosphere [41, 42].
Figure 3. FTIR spectra of the EtZnO NPs (1) and hydrozincite (2).
Download figure:
Standard image High-resolution image3.1.2. Optical and thermal characterization.
The electronic structure of ZnO NPs is characterized by the band gap (Eg), which is essentially the energy interval between the valence band (Ev) and the conduction band (Ec), each of which has a high density of states [28]. The generation of a specific type of ROS such as •OH, 1O2, or O2•– is governed by the metal oxide NPs related to the electronic structures as well as the redox potentials (EH) of different ROS generation reactions [28, 43, 44]. The oxidative stress induced by ZnO NPs is thought to be the main mechanism of their antimicrobial activity [1, 9, 28, 43–45]. Therefore, we calculated the electronic band gap energy (Eg) of EtZnO NPs because of their broad application in antimicrobial properties. The ZnO-NPs (10 μg ml−1) were dispersed in ethanol by using ultra sonication and then the solution was used to perform the UV–Vis measurement (figure 4(a)). The spectrum reveals a characteristic absorption peak of EtZnO NPs at wavelength of ∼A360nm which can be assigned to the intrinsic band-gap absorption of EtZnO NPs due to the electron transitions from the valence band to the conduction band (O2p → Zn3d) (figure 4(a)) [46, 47]. The sharp absorption peak of EtZnO NPs also indicated the narrow nanosize particle distribution. Moreover, the egg albumen showed the absorbance at wavelength of ∼A280nm due to the presence of the proteins in their composition (figure 4(a)). Thus, results indicate that the egg albumen does not influence the absorption of EtZnO NPs, suggesting that EtZnO NPs were fully functional. The electronic band gap (Eg) of the EtZnO NPs was determined by employing Tauc relationship as follows:
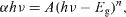
where α is the absorption coefficient (2.303A/t), h is Planck's constant, ν is the photon frequency, and Eg is the elctronic band gap. The value of n = 1/2, 3/2, 2 or 3 depending on the nature of the electronic transition responsible for absorption and n = 1/2 for direct band gap semiconductor. An extrapolation of the linear region of a plot of (αhν)2 on the y axis versus photon energy (hν) on the x-axis gives the value of the Eg as shown in figure 4(b). The Eg of EtZnO NPs was determined to be 3.55, which was the higher than bulk ZnO NPs powder (Eg =3.37 eV) [48]. The high value of the Eg is possibly attributed due to the quantum confinement effect of the NPs. The widening effect might be related to the influence of numerous factors such as structural parameters (size and pH), carrier concentrations and the presence of defects (oxygen vacancies), which may lead to the Burstein–Moss shift [49, 50].
Figure 4. EtZnO NPs optical and thermal characterizations. (a) Time dependent UV–visible absorption spectrum of EtZnO NPs synthesized by using egg albumen as biotemplate (1) and pure egg albumen (2). The inset of the figure 1(a) depicts the formation of the EtZnO NPs in the glass vial. (b) Tauc plot depicted the energy band gap of EtZnO NPs. (c) Concentration dependent fluorescence emission spectra of EtZnO NPs. (d) Graph illustrates the TGA and DTA based thermal behavior of EtZnO NPs.
Download figure:
Standard image High-resolution imageThe optical properties of ZnO are more interesting since confinement of charge carriers in the restricted volume of the small particles can lead to effects such as widening of Eg [51]. Since the EtZnO NPs have a wide Eg = 3.55 eV good electron transporting properties [52], they can be utilized as an anticandidal agent, which can efficiently kill the C. albicans via ROS production. The data suggest that the egg albumen facilitates the electronic band gap widening effect via controlling the nucleation and surface capping of the intermediate products (hydrogencite) [51]. The amino acid moiety in the egg albumen is sufficient to form proper capping, resulting in the formation of smaller sized EtZnO NPs, so as to keep the wide Eg [53].
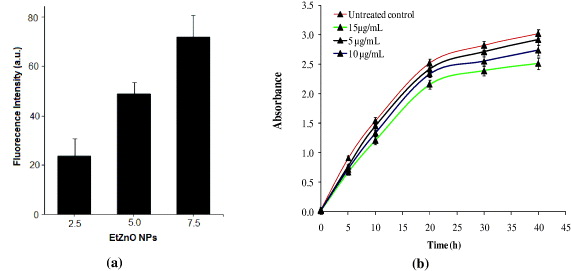
The photoluminescence behavior of EtZnO NPs could give information on energies and dynamics of photogenerated charge carriers as well as on the nature of the emitting states [54]. Figure 4(c) shows photoluminescence emission spectra in the visible range of EtZnO NPs and monitoring by measuring the dose-dependent changes in the intensity of EtZnO NPs. The emission spectra have a broad band with a maximum ∼532 nm which can be ascribed to the singly ionized oxygen vacancy with exited EtZnO NPs at ∼A370nm. The green emission in the visible region arises when a photogenerated hole (O–) trapped at a deep level above the valence band recombines with an electron trapped at a shallow level below the conduction band [55]. Usually, the emission intensity and band width are related to the size and nature of the carrier trapped states located at the surface of the nanocrystals [56]. From figure 4(d) we can see that about ∼5% of the total weight loss of EtZnO NPs might be due to the evaporation of water adsorbed on the surface of NPs [57, 58]. The differential thermal analysis (DTA) of EtZnO NPs shows the endothermic reaction peak at 135 °C possibly due to change of phases [59].
3.2. Anticandidal activity of EtZnO NPs
Before starting the experiments we determined the stability of EtZnO NPs (45 μg ml−1) in SD broth culture medium up to 40 h at 37 °C through the change in UV–visible absorbance characteristics. Significant change in agglomeration and absorbance of EtZnO NPs was not observed (figure 5(a)), suggesting that the SD broth culture medium does not significantly affect EtZnO NPs' stability, size and integrity. It was also observed that the colloidal solution of EtZnO NPs remained stable for 90 days and the significant change in the absorbance does not decrease (figure 5b). Similarly, no significant changes were found when surface modified NPs incubated in culture medium [37, 60].
Figure 5. EtZnO NPs stability in SD broth medium and during storage. (a) The stability of EtZnO NPs (45 μg ml−1) in the SD broth culture medium was monitored up to 40 h at 37 °C through the change in UV–visible absorbance characteristics. (b) Demonstrates the stability of the EtZnO NPs in MQ water during storage. The stability of EtZnO NPs in MQ water was monitored through the change in UV–visible absorbance characteristics. The bar graph illustrates that there was no significant change in the absorbance characteristics of EtZnO NPs (45 μg ml−1) up to the storage of 90 days at 25 °C.
Download figure:
Standard image High-resolution image3.2.1. Anticandidal activity of EtZnO NPs.
In light of the evidence, the rapid global spread of resistant in clinical isolates of C. albicans and new families of antimicrobial agents have a short life assurance, thus, the need to find new anticandidal agents is of supreme importance [61]. Researchers are increasingly turning their attention to nanomaterials, looking for new leads to develop better nano-antimicrobial drugs against MDR strains of C. albicans. In the present study we assessed the anticandidal activity of EtZnO NPs against MDR strain 077 of C. albicans. Anticandidal assays revealed that the EtZnO NPs efficiently suppressed the growth of C. albicans 077 in a dose dependent manner (figures 6(a)–(c)). The cells treated with the EtZnO NPs (15 μg ml−1) also exhibited cavity formation, examined by SEM analysis (figure 6(d)). These cavities possibly reflected the formation of apoptosome in the C. albicans 077 cells, indicating promising anticandidal activity. The untreated sample cells showed a normal pattern of growth with a lag phase of ∼4 h, active exponential phase of 8 to ∼21 h before attaining stationary phase. However, EtZnO NPs led to the suppression of growth and delay exponential phases of C. albicans 077 with minimum inhibitory concentration (MIC) ∼29.7 μg ml−1 (figure 6e), again proving strong antimicrobial activity of EtZnO NPs against C. albicans 077. The MIC is the lowest concentration of the compound at which the microorganism tested does not demonstrate visible growth. The almost complete cessation of growth was observed at 34.1 μg m1-1 concentration. The obtained anticandidal activity of EtZnO NPs is corroborated with previous published reports on anticandidal nanomaterials [62, 63].
Figure 6. Anticandidal activity of EtZnO NPs. (a) Zone inhibition and (b) in vitro killing of assays show the anticandidal activity of EtZnO NPs against C. albicans 077. (c) The graph shows the dose-dependent size of the zone of inhibition formed by EtZnO NPs. (d) SEM based observation of change in cell morphology of C. albicans 077, when treated with 15 μg ml−1 of EtZnO NP. (e) Growth curve analysis depicts the growth inhibition of C. albicans 077 in the presence of different concentrations of EtZnO NPs.
Download figure:
Standard image High-resolution image3.2.2. Role of ROS in anticandidal activity of EtZnO NPs.
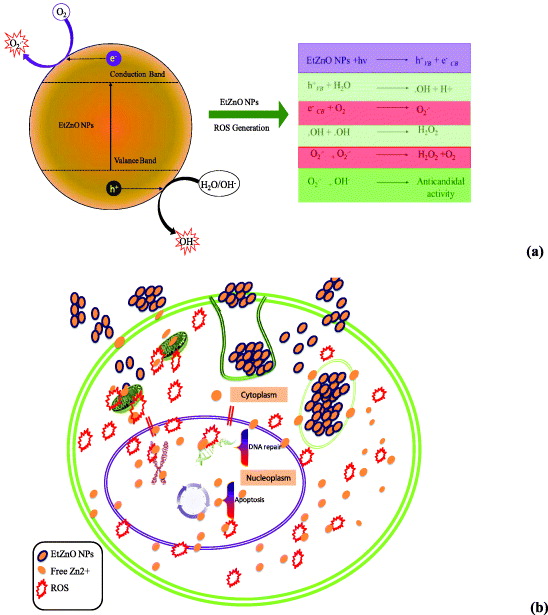
Recently, our group synthesized biosurfactant stabilized anticancer CdS QDs. This effect was associated with the production of ROS [37]. In light of these results, we concluded that the anticandidal effect of EtZnO NPs may be ROS dependent. As shown in figure 7(a), exposure of C. albicans cells to EtZnO NPs increased the intracellular ROS production in a time- and dose-dependent manner, when compared to untreated control. ROS-mediated oxidative stress is a well known inducer of cytotoxicity and apoptotic cell death in C. albicans [4]. Therefore, we sought to examine the possible role of ROS in anticandidal activity of EtZnO NPs. C. albicans were pretreated with 5 mM of histidine, a known scavenger of hydroxyl radicals (•OH) and singlet oxygen (1O2). The data revealed that the histidine completely abrogates the antimicrobial property of EtZnO NPs (figure 7(b)). This clearly indicates that the anticandidal activity of EtZnO NPs is due to ROS production. The electronic band gap (Eg) structures of the metal oxides NPs with the redox potentials (EH) of the different ROS generation reactions have been proposed [23]. The metal oxide NPs when excited with energy higher than the Eg, the electrons (e-) of metal NPs were promoted across the band gap to the conduction band (Ec), which creates a hole (h+) in the valence band (Ec). The electrons in the Ec and holes in the Ev exhibit high reducing and oxidizing power, respectively [28, 64]. The e- reacted with molecular oxygen to produce superoxide anion (O2•–) through reductive reactions. The h+ can extract electrons from water and/or hydroxyl ions to generate •OH (figure 8(a)). Taken together, results have confirmed that the ROS principally contributes the anticandidal activity of the EtZnO NPs (figure 8(b)). However, various research articles in the last five years have been published on generation of ROS by various metal-oxide NPs. The literature survey revealed that very limited research has been done on the role of the electronic band gap property of metal-oxide NPs in ROS generation [28, 65].
Figure 7. Role of ROS in anticandidal activity of EtZnO NPs. Determination of the ROS production within the C. albicans 077 cells treated with the different doses of EtZnO NPs. (b) Growth curve analysis shows the ROS quenching effect of histidine (5 mM) in C. albicans 077 cells, resulting in abrogate the antimicrobial property of EtZnO NPs, however, growth inhibition was recorded in the presence of EtZnO NPs alone, suggesting the involvement of ROS in anticandidal activity of EtZnO NPs.
Download figure:
Standard image High-resolution imageFigure 8. Plausible mechanistic aspect of the ROS generation and anticandidal activity of EtZnO NPs. (a) Plausible mechanistic aspect of the ROS generation induced by EtZnO NPs and their role in anticandidal activity. (b) Hypothetical anticandidal mechanism of EtZnO NPs against C. albicans 077. EtZnO NPs can enter into the cell by diffusion or endocytosis. Once EtZnO NPs are inside the cytoplasm, they can interfere with energy production in mitochondria and promote the generation of ROS. ROS and Zn+ ions released from EtZnO NPs may cross the nuclear membrane and cause DNA damage. DNA damage can be either repaired or lead to irreversible chromosome damage or cell death.
Download figure:
Standard image High-resolution image4. Conclusion
We examined that EtZnO NPs exhibit strong anticandidal activity against C. albicans 077 by alleviating ROS-mediated oxidative stress. An in depth understanding on energy band gap would definitely allow us to tailor new antimicrobial metal-oxide nanomaterials and effectively reduce experimental testing cost. In future, we suggest in-depth in vitro and in vivo studies that will help to identify anticandidal potential of EtZnO NPs which can be utilized in the management of diseases caused by MDR strains of C. albicans.
Acknowledgments
Financial support for this work through the Centre of Excellence in Materials Science (Nanomaterials), Department of Applied Physics, Zakir Hussain College of Engineering and Technology, Aligarh Muslim University, Aligarh-202002, Uttar Pradesh, India is greatly acknowledged. We are also grateful for the technical support of Mr Khateeb Ahmad, Lab Assistant.