Abstract
In this study a metal-organic framework (MOF-199) has been synthesized by solvent-thermal method. The conditions of preparation and activation processes have been investigated. The obtained material was characterized by methods of x-ray diffraction (XRD), infrared (IR) spectroscopy, thermogravimatric analysis (TGA) and scanning electron microscopy (SEM). The CO2 adsorption measurements were carried out on a high pressure volumetric analyzer (Micromeritics HPVA − 100). According to experimental results, Cu(NO3)2·3H2O has been shown to be the best copper(II) precursor for the synthesis of MOF-199 and N,N-dimethylformamide (DMF):C2H5OH:H2O with the ratio of 1:1:1 has been chosen as the most suitable solvent. The appropriate activation condition has been determined as follows: activate at 200 °C for 5 h and use CH3OH as the solvent to remove DMF. At the optimal conditions, an octahedral shape and three-dimensional (3D) structure of crystallite of MOF-199 was obtained. The synthesized MOF-199 expressed a high value of specific surface area (1448 m2 g−1 by Brunauer–Emmet–Teller (BET) method and 2028 m2 g−1 by Langmuir method) with Ta porous size of crystal of 11.8 Å and specific volume of 0.693 cm3 g−1; it was still stable up to 332 °C and its CO2 storage capacity reached to 206.59 cm3 (STP) g−1 at 25.76 bar.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metal-organic frameworks (MOFs) are new materials having very high values of specific surface area, normally about several thousand square meters per gram, for example, the specific surface area of material MOF-210 has been found 6240 m2 g−1 [1]. Therefore, MOFs have attracted an increasing interest from academic and industrial researchers in the last few years [2,3]. In addition to the synthesis and structural studies of MOFs, scientists have been interested in exploring the application of MOFs in different areas, such as gas storage, adsorption, gas separation, catalysis, drug storage and delivery, as well as luminescent and fluorescent materials, magnetic material, etc [1–4]. Nowadays, the warning phenomenon caused by carbon dioxide emission is considered as one of the most crucial problems of global environment protection [5]. Removal of CO2 from flue gas, synthetic gas and other industrial gases is usually carried out by absorption processes using amine-based materials, which should suffer inherent regeneration cost and inefficiency [6]. Other methods for CO2 uptake are based on adsorption by porous materials such as silicates, activated carbons and membranes. However, in order to provide long-term viability in CO2 removal, any adsorption system should combine two features: (i) the structure of adsorbent in the periodical cycle of CO2 uptake and release must be unchanged and (ii) the flexibility with which chemical functionalization and molecular-level fine-tuning can be achieved for optimized uptake capacities [7]. Many MOFs have been synthesized and tested for CO2 adsorption. According to Millward and Yaghi [7], the CO2 uptake capacity of MOF-177, IRMOF-1 and MOF-2 are of 33.5, 22.5, and 2.8 mmol g−1, respectively. Ni(HBTC)BPY synthesized from 1,3,5-benzenetricarboxylic acid, 4,4'-bipyridine and Ni(NO3)2·6H2O possessed a CO2 adsorption capacity of 108 cm3 g−1 [8]. CO2 uptake capacity of MOF-199 at low pressure (220 °C, 1 atm) was 69 ml g−1 as measured by Xie et al [9]. The maximum uptake of CO2 at 25 °C and high pressure ranges from 8 mmol g−1 as measured by Wang et al [5] to 11 mmol g−1, measured by Millward and Yaghi [7].
In this study the synthesis of the highly porous metal-organic framework MOF-199 is reported. The effect of parameters such as copper's salts, solvent volume, nature of exchanged solvents, activation temperature and reaction time were studied. The CO2 storage capacity of the material was also investigated.
2. Experimental
2.1. Synthesis of MOF-199
MOF-199 was prepared by a modification of the synthesis procedure described by Yaghi and co-workers [10] as follows: the mixture including 1.36 mmol of different copper (II) salts and 1,3,5-benzenetricarboxylic acid (0.84 mmol) was dissolved in N,N-dimethylformamide (DMF), C2H5OH and H2O (with ratio DMF:C2H5OH:H2O = 1:1:1) in a 20 ml vial. The vial was tightly capped and heated at 85 °C for 24 h to becoming blue crystal. The solid product then was removed by decanting with mother liquor and washed with DMF. After that solvent exchange was carried out with CH2Cl2 or CH3OH. The product was activated under atmospheric pressure condition (10−2 Torr).
In order to determine the optimal conditions for MOF-199 preparation different precursors Cu(NO3)2, CuCl2 and Cu(CH3COOH)2 were used; the volume of solvent varied from 6 to 12 ml. Activation time varied in the range of 8–15 h at 175 °C and in the range of 2–10 h at 200 °C.
2.2. Determination of physico-chemical properties
The morphology of the synthesized products was obtained by using a scanning electron microscope (SEM, JEOL 7401). Powder x-ray diffraction (XRD) data was recorded on a Bruker D8 Advance Diffractometer. Also, the thermogravimetric analysis (TGA, TA—Q200) of samples was performed to determine the thermostability of the materials. BET and Langmuir surface areas and total pore volumes of the samples were determined from N2 adsorption isotherms at 77 K on a Quantachrome NovaWin machine. Fourier transform infrared (FTIR) spectra (4000–400 cm−1) were obtained from KBr pellets using a Bruker Vector 22 instrument.
2.3. CO2 adsorption isotherms
The CO2 adsorption capacity of MOF-199 was investigated using a high pressure volumetric analyzer (Micromeritics HPVA—100). Heat of adsorption (ΔH) was calculated by equation [11]

where R is the gas constant (R = 8.314 J mol−1 K−1), T is temperature (T = 304 K) and n is the adsorbed amount (mol).
3. Results and discussion
3.1. MOF-199 synthesis
3.1.1. Influence of copper (II) precursors.
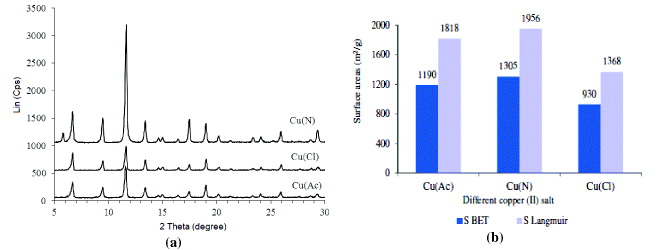
XRD patterns of MOF-199, synthesized from different copper (II) salts (figure 1(a)) are similar. Characteristic peaks of MOF-199 (at 2θ = 6.9°, 9.5°, 11.6°, 13.4°, 17.5°, 19.0° [5,11–14]) appeared in XRD patterns. Compared to MOF-199 prepared from CuCl2 and Cu(CH3COOH)2, the sample obtained from Cu(NO3)2 precursor possessed stronger characteristic peaks. In addition, as seen in figure 1(b), this sample was characterized by the highest value of specific surface area (SLangmuir = 1903 m2 g−1 and SBET = 1305 m2 g−1), much higher compared to the other ones. So, Cu(NO3)2 has been shown to be the best precursor for the synthesis of MOF-199.
Figure 1. XRD patterns (a) and specific surface areas (b) of the MOF-199, synthesized from different copper (II) precursors: Cu(Ac) denotes Cu(CH3COO)2·H2O; Cu(N)–Cu(NO3)2·3H2O; Cu(Cl)–CuCl22·H2O (Conditions: 9 ml of solvent C2H5OH:DMF:H2O = 1:1:1, exchanged solvent:CH2Cl2.)
Download figure:
Standard image High-resolution image3.1.2. Effect of solvent quantity.
As shown in figure 2(a), although the used amount of solvent did not lead to any changes in XRD spectrum of MOF-199, it significantly affected the intensity of characteristic peaks. When 9 ml of solvent was used, the obtained sample possessed the strongest intensity of characteristic peaks (figure 2(a)) and the largest specific surface area (figure 2(b)). One can suggest that a very small volume of solvent (6 ml), being not enough to neutralize all -COOH groups in the process of MOF creation, resulted in lower performance of crystals. However, when a large amount of solvent (12 ml) was used, the crystallization extent of MOF decreased due to the low concentration of reactants.
Figure 2. XRD patterns (a) and specific surface areas (b) of the MOF-199, synthesized using different volumes of solvent C2H5OH:DMF:H2O = 1:1:1. (Copper precursor: Cu(NO3)2·3H2O, exchanged solvent: CH2Cl2.)
Download figure:
Standard image High-resolution image3.1.3. Effect of activation temperature and activation time.
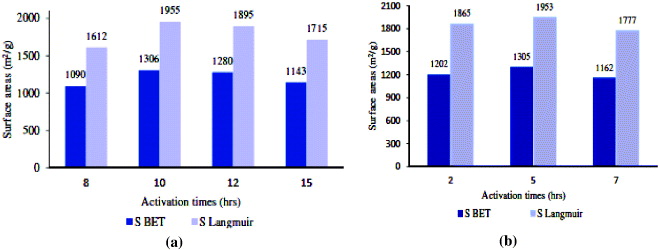
As seen in figure 3, the common trend in specific surface area of product is increasing with activation time, reaching a maximum at a certain value and then going down. According to figure 3, optimal activation time is reduced from 10 h down to 5 h when activation temperature increases from 175 to 200 °C. The samples obtained at the optimal activation times in both cases have approximately the same and the highest values of specific surface area (SBET = 1306 m2 g−1 and SLangmuir = 1955 m2 g−1), but these values are still lower than the value declared by Yaghi and co-workers [10]. This could be explained by the fact that in the obtained samples some amount of solvent (DMF) still remained in the structure of MOF-199.
Figure 3. The dependence of surface areas of MOF-199 on activation time at activation temperature of 175 °C (a) and 200 °C (b) (Copper precursor:Cu(NO3)2·3H2O, 9 ml of solvent C2H5OH:DMF:H2O = 1:1:1 and exchanged solvent: CH2Cl2.)
Download figure:
Standard image High-resolution image3.1.4. Effect of exchanged solvents.
It has been indicated that at the optimal conditions and using CH2Cl2 as exchanged solvent, the highest values of specific surface area of 1305 m2 g−1 by BET method and 1953 m2 g−1 by Langmuir method were observed. Using CH3OH as exchanged solvent instead of CH2Cl2, the specific surface area of MOF-199 increased and reached SBET = 1448 m2 g−1 and SLangmuir = 2028 m2 g−1. The reason for this effect is lies in smaller molecular size and more suitable polarity of CH3OH in comparison with CH2Cl2, leading to more complete removal of DMF from the network of MOF.
The results of the above study allowed selecting the optimal conditions for the preparation of MOF-199 as follows: the copper(II) precursor is Cu(NO3)2, volume of solvents DMF:C2H5OH:H2O = 1:1:1 is 9 ml, activation temperature and activation time are 200 °C and 5 h, respectively, and exchanged solvent is CH3OH. The physico-chemical characteristics of MOF-199 are introduced below.
3.2. Characteristics of MOF-199 prepared at the optimal conditions
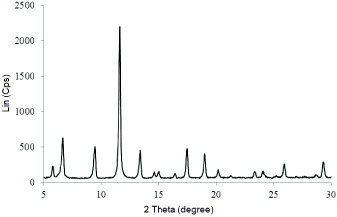
Powder XRD pattern for MOF-199 is shown in figure 4. The sample showed good crystallinity and the diffraction peaks matched well with already published XRD patterns of MOF-199 [10–15]. As follows from figure 4, and according to Biemmi [15], one can propose that there was no Cu2O existence in MOF-199. Crystalline size of MOF-199, calculated by the Sherrer equation [16] was 26 nm.
Figure 4. XRD pattern of obtained MOF-199.
Download figure:
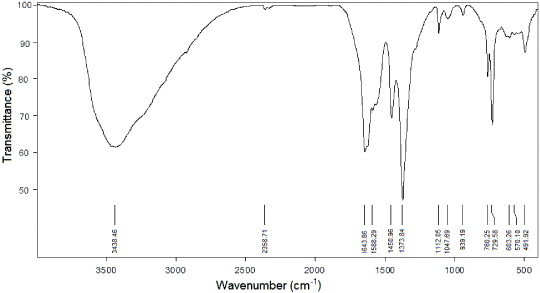
Standard image High-resolution imageAll the vibration bands of IR spectra (figure 5) were in good agreement with the published data on MOF-199 [11]. The IR spectra of the MOF-199 exhibited the strong stretching vibration of carboxylate anions at 1643.86 cm−1, proving the existence of the reaction of –COOH groups in the 1,3,5-benzenetricarboxylic acid with metal ions. The appearance of a broad band at 3500–2700 cm−1 indicated the presence of water and –OH groups in the structure of the material.
Figure 5. IR spectra of obtained MOF-199.
Download figure:
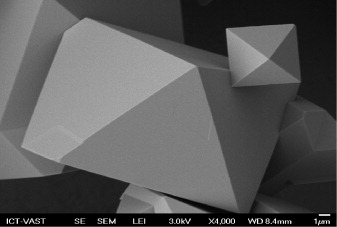
Standard image High-resolution imageAs follows from the SEM image (figure 6) the crystals of MOF-199 exist in the form of octahedral shape. The synthesized MOF-199 expressed high surface area (SBET = 1448 m2 g−1 and SLangmuir = 2028 m2 g−1); the porous size of 11.8 Å and the specific volume of 0.693 cm3 g−1 were observed. The surface properties of synthesized MOF-199 were very positive. The specific surface area and porous volume are equivalent to that of MOF-199 obtained by Yaghi and co-workers [10], Liu et al [17] and much higher than the values obtained by Li and Yang [11], Chowdhury et al [12] and Chui et al [18].
Figure 6. SEM image of the obtained MOF-199.
Download figure:
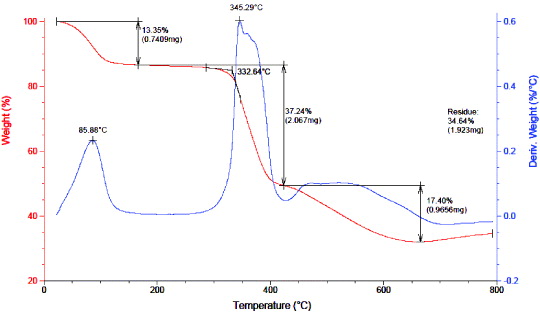
Standard image High-resolution imageAs is common for solid materials, the thermostability of the MOF-199 should be investigated. A differential thermogravimetric (DTG) diagram of MOF-199 (figure 7) had two main weight changes. The first weight loss of 13.35%, occurred between 30° and 180 °C due to vaporization of solvent (C2H2Cl2 and DMF), residing in the pore. The second step of weight loss was 54.64% in the temperature range of 332.64–660 °C due to decomposition of the material. So, MOF-199 is stable at temperature as high as 332.64 °C.
Figure 7. DTG diagram of the obtained MOF-199.
Download figure:
Standard image High-resolution image3.3. CO2 storage capacity of MOF-199
Figure 8(a) reported the isotherms of carbon dioxide adsorption performed at 30 °C in the range from 0.9032 to 30 bar for MOF-199. The carbon dioxide storage capacity of the synthesized MOF-199 reached to 206.59 cm3 (STP) g−1 at pressure of 25.76 bar, while the value previously reported by Yaghi was 246.4 cm3 (STP) g−1 at 45 bar and 25 °C [7]. The reason for this difference may lie in the difference of adsorption conditions. However, the carbon dioxide uptake of the synthesized sample in this study was higher than the reported data in [5, 19].
Figure 8. Adsorption and desorption isotherms (a) and heat of adsorption of CO2 on MOF-199 (b).
Download figure:
Standard image High-resolution imageThe dependence of adsorption heat of CO2 on volumetric uptake of CO2 calculated by equation (1) for MOF-199 is described in figure 8(b). It is notable that with increase of CO2 uptake from 50.46 to 206.59 cm3 g−1, the adsorption heat of CO2 decreased from 15.08 to 11.83 kJ mol−1.
4. Conclusion
From the experimental results one should conclude that the material MOF-199 has been successfully synthesized; the optimal conditions for its preparation have been identified. The synthesized sample MOF-199 possesses high value of surface area (SLangmuir = 2028 m2 g−1) with the porous size of crystal 11.8 Å, the specific volume 0.693 cm3 g−1 and a good thermostability (stable up to 332 °C). The synthesized MOF-199 should be considered as an effective adsorbent for CO2. The carbon dioxide storage capacity of the material reached 206.59 cm3 (STP) g−1 at pressure of 25.76 bar.
Acknowledgments
The research group acknowledge the financial support of the program code VAST 03.01/13-14 from The Materials Science Council, Vietnam Academy of Science and Technology and Professor Nguyen Van Hieu.