Abstract
In this work zeolites HY, HZSM-5 and mixes of zeolites with γ-Al2O3 in different ratios were taken as carriers for 0.8 wt% Pd catalysts. Physico-chemical characteristics of the catalysts were determined by methods of Brunauer–Emmett–Teller (BET)–N2 adsorption, x-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive x-ray spectroscopy (EDS), transmission electron microscopy (TEM), temperature-programmed reduction (TPR), hydrogen pulse chemisorption (HPC) and NH3 adsorption–desorption. The activity of catalysts was studied at 225–450 °C, at 0.1 and 0.7 MPa with molar ratio of H2:n-C6H14 = 5.92 and n-hexane concentration 9.2 mol%. Mixing of γ-Al2O3 with zeolite made acidity of catalyst weaken and led to a decrease of Pd cluster size, to an increase of Pd dispersity and a reduction of the extent of Pd in the case of catalyst Pd/HY; but for the catalyst Pd/HZSM-5 such mixing led to the reverse effect. That is why the increase of activity in the first case and the decrease of activity in the second case have been observed. It has been found that the optimal ratio of mixed carrier is γ-Al2O3:HY = 2.5:1 and the optimal calcined temperature of NH4ZSM-5 to obtain HZSM-5 is 500–550 °C. An increase of reaction pressure from 0.1 to 0.7 MPa remarkably increased the activity, selectivity and stability of Pd-based catalysts.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nowadays emission standards for gasoline strictly require the reduction of benzene, total content of aromatic hydrocarbons, olefins and sulfur. According to the Euro-3 standard (from 2000), the limit of olefins, aromatics and benzene contents are of 18, 42 and 1%, respectively. In 2005, when the Euro-4 standard began to take effect, the content of sulfur had to reduce to 50 ppm and the content of aromatic hydrocarbons to 35% [1]. Both standards, Euro-4 and Euro-5, require the benzene concentration in gasoline to not exceed 1 vol%. From the beginning of 2011, when the standard mobile source air toxics (MSAT II) began to take effect in Europe and in the United States, the total concentration of aromatic hydrocarbons and the partial concentration of benzene in gasoline were defined as not exceeding 25 and 0.62 vol%, respectively [2].
In order to increase octane number and reduce the content of aromatic hydrocarbons in gasoline, processes of alkylation and isomerization of light paraffins have been involved and applied in the refinery industry. Isomerizing process should boost the octane number in light naphtha fraction (boiling points up to 85 °C) about 15–20 units. Therefore, the isomerization reaction of light paraffins is attracting more and more attention from researchers.
So far several catalyst generations have been developed for the isomerizing process. Among these catalysts bifunctional contacts have been shown to be the most promising thanks to the balance of two functions—metallic and acidic. At the present time, the reaction of light paraffins isomerization is being conducted at high temperatures (225–302 °C) as well as at low temperatures (127–177 °C). In the first case catalysts based on noble metals supported on zeolites with high tolerance to impurities and relatively long lifetime are applied [3]. In the second one, catalysts based on platinum supported on chlorinated alumina are utilized. Catalysts of this kind, although giving high yields in the formation of isoparaffins at low temperatures, are very sensitive to impurities [4]. Palladium is cheaper than platinum and the choice of Pd as an alternative to Pt active component, is determined on the basis of its performance and stability.
The size of zeolite pores plays a determining role in products selectivity. According to Dilson and co-workers [5], as carriers, zeolites HY with pore size up to 12.7 Å are favorable for the operating catalysts to produce two-branched isomers of isohexane, which are characterized by high octane number. Nevertheless, with high acidity, HY zeolites also are favorable for cracking reaction (in these wide pores), leading to lowering the isomerizing process. In replacement of HY zeolite HZSM-5 was selected. This zeolite with pore size less than 6 Å is characterized by two types of channels: straight ten-ring channels running parallel to the corrugations (0.51 nm × 0.55 nm) and sinusoidal ten-ring channels perpendicular to the sheets (0.54 nm × 0.56 nm). The structure and size of this pore system are suitable for conversion of naphtha fraction, containing paraffinic hydrocarbons with carbon number C4 to C10, with high geometric selectivity, especially in isomerization reaction. Besides, HZSM-5 zeolites are characterized by high value of Si/Al, strong acidity that strengthens the conversion of hydrocarbon including isomerization. Okuhara [6] conducted n-hexane isomerization on catalysts Pt/HZSM-5 with platinum concentrations, ranging from 0.6 to 1.2 wt% at the temperature range 280–340 °C and reached conversion extents of about 77% with values of selectivity around 98%. Al2O3 is considered as a suitable carrier for isomerization reaction, but characterized by weak acidity. It is probable that the combination of alumina and zeolite should lead to a kind of carriers, possessing appropriate acidity for the given reaction.
In our previous works [7,8] the Pd catalysts supported on mixed carriers, comprising cation–decationized forms of Y-type zeolite and aluminum oxide in n-hexane isomerization at atmospheric pressure has been studied. It has been found that optimal Pd concentration is 0.8 wt% and appropriate value of zeolite:alumina (CaHY-80–18:Al(OH)3) ratio was 1:4. At this composition of catalyst the yield of isohexane was highest. In this paper we report the results, obtained in our investigation of the replacement of Pt with Pd in n-hexane isomerization, proceeding on bifunctional catalysts. For carriers preparation, zeolites HY and HZSM-5 were taken to mix with γ-Al2O3 and the reaction was carried out at atmospheric pressure and at 0.7 MPa.
2. Experimental
Aluminum oxide was prepared by coprecipitating 5%-solution of ammonia with solution of Al(NO3)3.9H2O up to pH = 8–9. The precipitate was aged 12 h and the product Al(OH)3 then was washed by distilled water, dried and calcined at 500 °C for receiving γ-Al2O3. (NH4)ZSM-5 (Zeolist International (USA)) was calcined at 400–550 °C for 3 h to obtain HZSM-5. Mixed carriers were obtained by mechanical mixing of Al(OH)3 with HY or HZSM-5, and then calcined at 500 °C for 6 h.
Pd (0.8 wt%) was loaded into the catalyst by impregnation method, then dried and calcined at 400 °C for 3 h. Catalysts were assigned as followed: Pd/HZSM-5-500 means 0.8 wt% of Pd on (NH4)ZSM-5 calcined at 500 °C; Pd/Al-HZSM-5(2:1) means 0.8 wt% of Pd on mixed carrier γ-Al2O3 and HZSM-5-500 with weight ratio Al2O3:zeolite = 2:1.
Physico-chemical properties of the catalysts were characterized by methods of Brunauer–Emmett–Teller (BET)–N2 adsorption, x-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive x-ray spectroscopy (EDS), transmission electron microscopy (TEM), temperature-programmed reduction (TPR) (in temperature range from room temperature to 550 °C), hydrogen pulse chemisorption (HPC), and NH3 adsorption–desorption. Before reaction the catalysts were activated in a flow of hydrogen with the flow velocity of 4 l h−1 during 2 h at 0.1 MPa and 400 °C.
Activity of the catalysts in n-hexane isomerization was determined in a microflow reactor at atmospheric pressure and at 0.7 MPa, the reaction temperature ranging from 225 °C to 450 °C; the flow velocity was 7.5 l h−1, catalyst weight 1.5 g, mole ratio H2:n-C6H14 of 5.92, n-hexane concentration was 9.2 vol %. The reaction mixture was analyzed on the Gas Chromatograph Agilent Technologies 6890 Plus with an FID detector, DB 624 column with 30 m of length and 0.32 mm of outer diameter was used.
3. Result and discussion
3.1. Catalysts carried on zeolites HZSM-5 and HY
3.1.1. Physico-chemical properties of catalyst.
As seen in figure 1, XRD patterns of catalysts Pd/HZSM-5 and Pd/HY are the same as HZSM-5 and HY, respectively. Particle size of carrier can be calculated by the following equation [9]:

where ρ (g cm−3) is the density of carrier (ρ of HZSM-5 is 0.45 g cm−3, of HY is 0.48 g cm−3 and of γ-Al2O3 is 0.92 g m−3), SBET (m2 g−1) is the specific surface area.
Figure 1. XRD patterns of catalysts. (a) XRD patterns of zeolites and catalysts: 1—HZSM-5; 2 — Pd/HZSM-5-400, 3—Pd/HZSM-5-450, 4—Pd/HZSM-5-500, 5—Pd/HZSM-5-550; 6—zeolite HY; 7—Pd/HY. (b) XRD patterns of catalysts on mixed carriers: 1—Pd/Al; 2—Pd/Al-HZSM-5-500(1:1); 3—Pd/HZSM-5; 4—Pd/Al-HY(2.5:1); 5—Pd/HY.
Download figure:
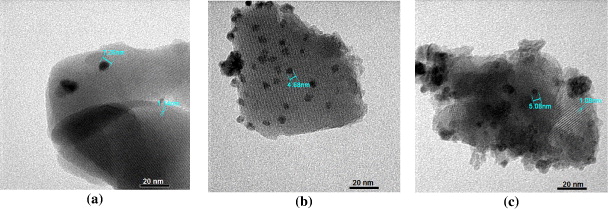
Standard image High-resolution imageIn figure 2 one can see rectangular cubic crystallites of zeolites with dimensions 200–260 and 400–600 nm, respectively, for catalysts Pd/HZSM-5 and Pd/HY. As follows from table 1, for catalyst Pd/HZSM-5 the calcination temperature did not influence remarkably physico-chemical characteristics of zeolite phase; values of both the quantities dZeol and d varied in ranges of 31.5–32.2 and 41.9–44.7 nm, respectively. Also, the changes in values of specific area were not significant. Nevertheless, it is notable that with increase of calcination temperature from 400 °C to 550 °C the dispersity of Pd improved (increased from 6.29 to 28.19%) and the value of Pd cluster size reduced from 18.4 to 4.1 nm. Pd cluster size (dPd) calculated by HPC and measured by TEM are relatively close; on Pd/HZSM-5-500, dPd is 5 nm by HPC and 7.36 nm by TEM (figure 3). Catalyst Pd/HY calcined at 550 °C is characterized by a higher value of surface area. On this catalyst the determined values by HPC of Pd cluster size and Pd dispersity are 7.3 nm and 15.95%, respectively. Thus, compared to Pd/HZSM-5, catalyst Pd/HY possesses higher value of surface area but is characterized by a worse dispersity of supported metal. The reason may be included in wider pore size and weaker acidity of faujasite-type zeolite that should lead to a weaker interaction between metal and carrier than in the case of Pd on HZSM-5. In study [10] on catalysts 0.88 wt% Pd supported on ZrO2 and WO3-promoted ZrO2, the values 2.93 nm for quantity dPd and 4.8 and 3.8 for quantity γPd, respectively, were observed.
Figure 2. SEM images of Pd catalysts supported on different carriers. (a) Pd/HZSM-5-400, (b) Pd/HZSM-5-500, (c) Pd/HY-550, (d) Pd/Al, (e) Pd/Al-HZSM-5(1:1), (f) Pd/Al-HY(2.5:1).
Download figure:
Standard image High-resolution imageFigure 3. TEM images of Pd catalysts. (a) Pd/HZSM-5-500, (b) Pd/Al-HZSM-5(1:1), (c) Pd/Al-HY(2.5:1).
Download figure:
Standard image High-resolution imageTable 1. Surface area (SBET); crystallite size of HZSM-5 calculated at 2θ = 7.9° and of HY calculated at 2θ = 6.5° (dzeol); particle dimension of zeolites calculated by equation (1) (d); Pd clusters size (dPd) and Pd dispersity (γPd) determined by HPC; and results of elemental analysis calculated by energy dispersive x-ray spectroscopy (EDS).
| Catalysts | SBET (m2 g−1) | dZeol (nm) | d (nm) | dPd (nm) | γPd (%) | Elemental analysis (atom%) | |||
|---|---|---|---|---|---|---|---|---|---|
| O | Si | Al | Pd | ||||||
| Pd/HZSM-5-400 | 306.6 | 32.1 | 43.5 | 18.4 | 6.29 | 48.70 | 47.60 | 3.17 | 0.52 |
| Pd/HZSM-5-450 | 318.0 | 31.6 | 41.9 | 7.5 | 15.34 | – | – | – | – |
| Pd/HZSM-5-500 | 298.0 | 31.5 | 44.7 | 5.0 (7.36a) | 23.30 | 43.44 | 53.34 | 2.95 | 0.27 |
| Pd/HZSM-5-550 | 301.8 | 32.2 | 44.2 | 4.1 | 28.19 | – | – | – | – |
| Pd/HY-550 | 409.0 | 33.1 | 29.3 | 7.3 | 15.95 | 33.90 | 48.03 | 16.85 | 1.24 |
As seen in EDS images (figure 4), Pd is distributed on catalyst surface fairly evenly. The average values of element distribution on catalyst surface is given in table 1. For catalysts Pd/HZSM-5 the values of Si/Al on surface are fairly high; after calcination at 400 °C the value of this ratio was about 15, but calcination at 550 °C made the ratio Si/Al obtain a value of about 18. For catalyst Pd/HY-550 the value of ratio Si/Al was only about 3.
Figure 4. EDS images of samples (a) Pd/HZSM-5-500, (b) Pd/HY-550, (c) Pd/Al-HZSM-5(1:1), (d) Pd/Al-HY(2.5:1). (The color of elements: Si—red; Al—blue; Pd—green.)
Download figure:
Standard image High-resolution imageAccording to [11], for both Pd/Al and Au–Pd/Al samples, the presence of TPR peaks at about 81 °C indicates the reduction of PdO species interacting with the alumina surface. In addition, the TPR profile of the Au–Pd/Al sample shows a peak at 31 °C, indicative of the reduction of bulk PdO. The negative peak of H2 consumption at 84 °C is attributed to H2-desorption from the decomposition of a bulk palladium hydride formed through H-diffusion within the Pd crystallites [12]. So, TPR diagrams (figure 5(a)) of all Pd/zeolite catalysts had only one peak with Tmax = 65–80 °C, which characterizes the reduction of PdO species interacting with the carrier surface. As follows from table 2, reduction extent of PdO is increasing with calcination temperature of NH4ZSM-5. Samples Pd/HZSM-5–500 and Pd/HZSM-5–550 have approximately the same and the highest value of reduction extent, and this value is higher than that of Pd/HY catalyst.
Figure 5. TPR diagrams of catalysts. (a) 1—Pd/HZSM-5-400; 2—Pd/HZSM-5-500; 3—Pd/HZSM-5-550; 4—Pd/HY. (b) 1—Pd/Al; 2 —Pd/Al-HZSM-5(2:1); 3—Pd/Al-HZSM-5(1:1); 4—Pd/Al-HY(2.5:1); 5—Pd/Al-HY(1:1).
Download figure:
Standard image High-resolution imageTable 2. Maximal reduction temperature (Tmax), reduction extent (KRed) and acidity of catalysts.
| Catalysts | Tmax (°C) | KRed (%) | Acidity (mmol NH3 per 100 g catalyst) | |||
|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | |||
| Pd/HZSM-5-400 | 75 | 31.33 | – | – | – | – |
| Pd/HZSM-5-450 | – | – | 8.544 | 19.384 | 5.142 | 33.070 |
| Pd/HZSM-5-500 | 75 | 35.50 | 8.002 | 18.430 | 6.785 | 33.217 |
| Pd/HZSM-5-550 | 80 | 35.76 | 5.531 | 14.498 | 12.696 | 32.725 |
| Pd/HY-550 | 65 | 29.84 | 9.369 | 8.872 | 7.160 | 25.401 |
Results of acidity determination indicate that catalysts Pd/HZSM-5 possess higher total acidity compared to catalyst Pd/HY (∼ 33 mmol NH3 compared to 25.4 mmol NH3 per 100 g catalyst). Both catalysts Pd/HZSM-5-500 and Pd/HY-550 are characterized by closed values of strong acidity. However, in values of medium acidity catalyst Pd/HY-550 is characterized only by figure of 8.9 mmol per 100 g catalyst, then catalyst Pd/HZSM-5-550-18.4 mmol per 100 g catalyst.
3.1.2. Activity and selectivity of catalysts.
On all the catalysts a common phenomenon can be observed: when reaction temperature increased the conversion of n-hexane increased but the selectivity in isohexane decreased, so for each catalyst the yield of main product must obtain maximal value at a certain temperature. At pressure 0.1 MPa, for catalysts Pd/HZSM-5 optimal temperatures of the reaction were observed in the range 250–275 °C and for catalyst Pd/HY-550 optimal temperature was 350 °C.
Table 3 shows activity and selectivity data of the studied catalysts at their optimal temperatures at 0.1 MPa. The reaction products comprise unreacted n-hexane, isomers of isohexane, such as 2,3-dimethyl butane (2,3- DMB), 2-methyl pentane (2-MP), 3-methyl pentane (3-MP) and products of cracking.
Table 3. Catalysts supported on zeolites: n-hexane conversion (X), selectivity in isohexane (Si−C6), isohexane yield (Yi−C6), 2,3-DMB:2-MP:3-MP ratio, cracking selectivity (Scr) and octane number of liquid product (RON) at optimal temperatures (Topt) and at atmospheric pressure.
| Catalysts | Topt (°C) | X (%) | Si−C6 (%) | Yi−C6 (%) | 2,3-DMB:2-MP : 3-MP | Scr (%) | RON |
|---|---|---|---|---|---|---|---|
| Pd /HZSM-5-400 | 250 | 44 | 87 | 39 | 1:50:23 | 13 | 42.0 |
| Pd/HZSM-5-450 | 275 | 31 | 33 | 10 | 1:46:19 | 67 | 31.4 |
| Pd/HZSM-5-500 | 275 | 66 | 76 | 50 | 1:23:12 | 24 | 58.5 |
| Pd/HZSM-5-550 | 275 | 53 | 93 | 50 | 1:32:17 | 7 | 51.6 |
| Pd/HY-550 | 350 | 32 | 59 | 17 | 1:12:7 | 37 | 30.0 |
Calcination temperature of (NH4)ZSM-5 significantly affected the catalytic activity of Pd/HZSM-5. Among the considered catalysts, Pd/HZSM-5-400 and Pd/HZSM-5-450 are characterized by the lower activity, selectivity and isohexane yield. This can be explained by their lower reduction extent. At pressure 0.1 MPa, both samples Pd/HZSM-5-500 and Pd/HZSM-5-550 expressed approximately equally high efficiency in isohexane production probably due to their high reduction extent. As seen in table 3, the first sample expressed higher activity but lower selectivity compared to the second one. Two catalysts gave the same yield of isohexane (about 50%). Considering their acidity (table 2), one can see that the first sample possesses a greater number of medium acidic centers but fewer strong acidic centers than the second one; the values of total acidic centers on both the catalysts are identical. This fact indicates that acidic centers on carrier surface must play their role in activity and selectivity of catalysts for the given reaction. Besides, the ratio of 2,3-DMB: (2-MP + 3-MP) observed on sample Pd/HZSM-5-500 was the highest in comparison with that on other Pd/HZSM-5 catalysts. This is one of the reasons, leading to the highest RON value of the liquid product obtained on this catalyst. The cracking composition was C3–C5 hydrocarbons, in which the proportion of C3 was preferable. It means that the cracked hydrocarbon was broken at the center of the skeleton.
Compared to catalyst Pd/HY, catalysts Pd/HZSM-5 gave higher activity but much lower ratio of two-branched/one-branched isomers [2,3-DMB: (2-MP + 3-MP)]. This should be understandable, because catalyst Pd/HY is characterized by lower acidity, bigger cluster dimension and worse dispersity of Pd and lower reducibility, but much wider pore size than Pd/HZSM-5. As indicated above, pore size of zeolite HY is up to 1.2 nm, and pore size of HZSM-5 is less than 0.6 nm, while diameters calculated by Lennard–Johns for n-C6H14 is 0.43 nm, for 2-MP is 0.50 nm and for 2,2-DMB is 0.62 nm.
3.2. Catalysts on mixed carriers Al2O3 + Zeolite
For zeolites characterized by high acidity, bifunctional catalysts supported on zeolites express high selectivity for cracking reaction. In order to reduce the acidity of zeolites to be suitable for isomerization reaction, γ-Al2O3 with lower acidity has been taken to add (mix) to zeolites (HY-550 and HZSM-5–500) for preparation of mixed carriers [7,8].
3.2.1. Physico-chemical properties of catalysts.
The analysis of results on XRD (figure 1(b), line 1) and SEM images (figure 2(d)) indicates that aluminum oxide exists in amorphous phase like fine loose cotton with particle size in the range 33–40 nm. XRD patterns of catalysts supported on mixed carriers Pd/Al-HZSM-5 and Pd/Al-HY (figure 1(b)) are similar to those of Pd catalysts supported on pure zeolites (figure 1(a)). Characteristic peaks of zeolite HZSM-5 (at 2θ = 7.9°, 9°, 14.8°, 15.6°, 16°; 23.3°, 23.9°, 24.4°, 29.3°, 30.1° degrees etc) and of zeolite HY (at 2θ = 6.5°, 10.5°, 12°, 16°, 19°, 21°, 24°, 27.5°, 32°) also appeared in XRD patterns of catalysts on mixed carriers but with weaker intensities. Also, the ratio Al2O3:zeolite does not influence the characteristics of XRD patterns. Besides, the SEM image of catalyst on mixed carrier (figures 2(e) and (f)) is similar to that of catalyst on pure zeolite (figures 2(a)–(c)). In figures 2(e) and (f) one can see rectangular cubic crystallites of zeolites with dimensions 120–300 nm and 300–500 nm respectively for catalysts Pd/Al-HZSM-5 and Pd/Al-HY on loose alumina. Thus, from analysis of the obtained results it should be concluded that the structure of zeolites HZSM-5 and HY in mixed carriers was not subject to change.
It is interesting to note that, according to EDS data (figures 4(c) and (d) and table 4), for catalysts supported on mixed carriers the values of ratio Si/Al was reduced; in several areas atomic composition of aluminum even exceeds that of silicon. It is possible to propose that on catalyst surface the interaction between aluminum oxide and zeolite is able to form different microphases, although, as confirmed by XRD data, the structure of zeolite was not subject to change.
Table 4. Physico-chemical properties of Pd catalysts supported on mixed carriersa.
| Catalyst | SBET (m2 g−1) | dZeol (nm) | dPd (nm) | γPd (%) | Elemental analysis (atom%) | |||
|---|---|---|---|---|---|---|---|---|
| O | Si | Al | Pd | |||||
| Pd/Al | 218 | – | 25.0 | 4.46 | 26.0 | 0 | 60.6 | 13.4 |
| Pd/Al-HY(3:1) | – | 33.6 | 6.2 | 18.69 | – | – | – | – |
| Pd/Al-HY(2.5:1) | 285 | 34.1 | 6.1 (5.08a) | 18.8 | 25.7 | 14.0 | 59.6 | 0.73 |
| Pd/Al-HY(1:1) | 322 | 27.8 | 4.4 | 26.1 | – | – | – | – |
| Pd/Al-HY(1:2) | – | – | 4.2 | 27.57 | – | – | – | – |
| Pd/Al-HZSM-5(2:1) | – | – | 6.2 | 18.74 | – | – | – | – |
| Pd/Al-HZSM-5(1:1) | 259 | 33.0 | 8.5 (4.68b) | 13.68 | 31.57 | 35.11 | 32.26 | 1.26 |
| Pd/Al-HZSM-5(1:2) | – | – | 10.5 | 10.99 | – | – | – | – |
aSymbols are similar to those in table 1. bTEM data.
Lower surface area of γ-Al2O3 compared to zeolites resulted in smaller SBET values of the catalysts on mixed carrier (table 4). Like pure zeolite carriers, mixed carriers are characterized by the same crystallites sizes of zeolite. Among catalyst samples in table 4, catalyst Pd on γ-Al2O3 possesses the highest value of Pd particle size and the lowest value of metal dispersity. It is noticeable that characteristics of Pd distribution on (HY + γ-Al2O3) carriers were better than those on (HZSM-5 + γ-Al2O3). Moreover, while on the first type carriers the Pd dispersion improved with zeolite content, on the second type carriers this quantity changed in opposite direction with zeolite content.
TPR diagrams of catalyst carried on Al2O3 and mixed carriers had only one peak with Tmax = 70–80 °C characterizing the reduction of PdO species interacting with the carrier surface (figure 5(b)). It should be noted that mixing of aluminum oxide to zeolite HY made the reduction extent of catalyst increase from 30% up to ∼ 34–42%, depending on the ratio Al2O3:HY. Also, on addition of Al2O3 to HZSM-5, this quantity reduced to be lower than that of catalysts Pd/Al-HY. This should be understandable, because catalyst Pd/Al-HZSM-5 is characterized by bigger cluster dimension and lower dispersity of Pd (table 4).
From results in table 5 one can see that catalyst Pd/Al is characterized by a very low acidity, much lower compared to catalysts Pd/HY and Pd/HZSM-5 (table 2). Generally, the acidity of catalyst on a mixed carrier is between the acidity of catalyst supported on aluminum oxide and the acidity of catalyst supported on zeolite and acidity is increasing with zeolite content. The acidity of Pd/Al-HZSM-5 (1:1) is equal to only half in total and one fourth in medium acidity of catalyst Pd/HZSM-5. Among catalysts supported on mixed carrier Al2O3 + HY, sample Pd/Al-HY (2.5:1) is characterized by a lowest acidity; its value is four times higher compared to that of Pd/Al and one third compared to Pd/HY. On this catalyst the value of medium acidity is three times higher, but the strong acidity is only 1.5 times higher than on Pd/Al. The total quantity of strong and medium acidity of catalyst Pd/Al-HY(2.5:1) is equal to one eighth of that on catalyst Pd/HY. Thus, the obtained results indicate that mixed carrier is able not only to produce catalyst with suitable acidity but also to control crystallites size and dispersion of the supported metal.
Table 5. Maximum reduction temperature (Tmax), reduction extent (KRed) and acidity of catalysts supported on mixed carriers.
| Catalyst | Tmax (°C) | KRed (%) | Acidity (mmol NH3 per 100 g catalyst) | |||
|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | |||
| Pd/Al | 75 | 34.21 | 0.964 | 0.445 | 0.751 | 2.160 |
| Pd/Al-HY(3:1) | 80 | 41.57 | 5.950 | 1.844 | 1.301 | 9.095 |
| Pd/Al-HY (2.5:1) | 70 | 41.20 | 5.832 | 1.290 | 1.080 | 8.202 |
| Pd/Al-HY (2:1) | 80 | 37.64 | 7.044 | 1.156 | 3.057 | 11.257 |
| Pd/Al-HY (1:1) | 70 | 41.89 | 6.922 | 2.264 | 2.397 | 11.583 |
| Pd/Al-HY (1:2) | 75 | 33.84 | 7.301 | 2.800 | 6.591 | 16.692 |
| Pd/Al-HZSM-5(2:1) | 80 | 27.40 | 6.600 | 1.430 | 1.910 | 9.940 |
| Pd/Al-HZSM-5(1:1) | 75 | 24.79 | 8.300 | 4.520 | 3.470 | 16.290 |
| Pd/Al-HZSM-5 (1:2) | – | – | 7.600 | 4.720 | 4.250 | 16.570 |
3.2.2. Activity and selectivity of catalysts.
Activity and selectivity of Pd catalysts supported on mixed carriers are presented in table 6.
Table 6. Activity of Pd-based catalysts at optimal temperatures (Topt) and 0.1 MPa.
| Catalysts | Topt(°C) | X (%) | Si−C6 (%) | Yi−C6 (%) | 2,3DMB: 2-MP : 3-MP | Scr (%) |
|---|---|---|---|---|---|---|
| Pd/Al | 400 | 18 | 92 | 16.7 | 1:100:57 | 8 |
| Pd/HY-550 | 350 | 32 | 59 | 18.9 | 1:12:7 | 37 |
| Pd/Al-HY(1:2) | 325 | 34 | 72 | 24.5 | 1:12:7 | 28 |
| Pd/Al-HY(1:1) | 325 | 23 | 77 | 17.7 | 1:14:8 | 23 |
| Pd/Al-HY (2:1) | 300 | 19 | 90 | 17.1 | 1:8:24 | 10 |
| Pd/Al-HY (2.5:1) | 325 | 38 | 94 | 35.7 | 1:12:6 | 6 |
| Pd/Al-HY (3:1) | 325 | 29 | 92 | 26.7 | 1:11:7 | 8 |
| Pd/HZSM-5-500 | 275 | 66 | 76 | 50.2 | 1:23:12 | 24 |
| Pd/Al-HZSM-5 (1:2) | 275 | 31 | 75 | 23.3 | 1:29:16 | 25 |
| Pd/Al-HZSM-5 (1:1) | 300 | 65 | 71 | 46.2 | 1:24:13 | 29 |
| Pd/Al-HZSM-5 (2:1) | 275 | 38 | 88 | 33.4 | 1:46:26 | 12 |
The reaction was carried out at 'optimal temperature' for each catalyst and pressure of 0.1 MPa. Data in table 6 indicate that, as a rule, catalyst Pd/Al is characterized by the lowest values of n-hexane conversion and isohexane yield and the highest optimal temperature compared to other catalysts. However, this catalyst expressed also the lowest cracking selectivity due to the lowest acidity. One can put the activity order of catalysts supported on single carriers as follows: Pd/HZSM-5-500 > Pd/HY-550 > Pd/Al. The order of optimal reaction temperatures for these catalysts is in the opposite direction. Among these catalysts, the highest values of conversion and main product yield were observed on Pd/HZSM-5, the highest isohexane selectivity belongs to Pd/Al, and Pd/HY gave the highest proportion of two-branched isomers. These results can be explained by the structure and properties of carriers as shown and interpreted above. One can notice a feature included in the distribution of cracking products on Pd/Al and on other catalysts. If on Pd/Al the content of C4 and C5 are predominant in products of cracking, on the rest of the catalysts, proportions of hydrocarbons C3:C4:C5 did not vary significantly.
The common trend in activity variation for catalysts supported on mixed carriers is increasing with zeolite content, reaching a maximum at a certain proportion of zeolite and then going down. It should be considered that optimal compositions for this kind of catalysts are as follows: Al2O3:HY = 2.5:1 and Al2O3:HZSM-5 = 1:1. This could be explained by the fact that in these catalysts the ratio between amount of metallic centers and acidic centers is reaching optimal value. Naturally, when the proportion of zeolite is growing, cracking selectivity increases and selectivity on isohexane reduces.
Catalyst with optimal composition Pd/Al-HY(2.5:1) gave higher values of n-hexane conversion, isomerization selectivity and isohexane yield compared to Pd/HY, while catalyst Pd/Al-HZSM-5(1:1) expressed lower activity compared to catalyst Pd/HZSM-5. This fact can be explained as follows: alumina in mixed carriers reduced the acidity of the obtained catalysts, but alumina created opposite effects for palladium properties on two types of catalysts. As seen above, on catalysts supported on alumina plus HY zeolite the effect is improvement of Pd dispersion (decrease of particle size, increase of dispersity) and reductibility, while on catalysts supported on alumina plus HZSM-5 zeolite, the effect is the reverse. In other words, addition of alumina to zeolite HY made the physico-chemical properties of catalysts change toward being favorable for isomerization reaction, while addition of alumina to zeolite HZSM-5 made these properties become worse for the given reaction. Since the addition of alumina to zeolites leads to decrease of catalyst acidity, it is understandable that herewith the stability of catalysts supported on mixed carriers should be better than that on catalysts supported on zeolites alone. At the given conditions the lifetime of catalyst Pd/Al-HY (2.5:1) was 23.7 h, while the lifetime of Pd/HY was only 1.25 h. The lifetime of Pd/Al-HZSM-5 (1:1) also was longer than that of Pd/HZSM-5 (1.5 h compared to 1.0 h).
In order to improve the activity, selectivity and stability of catalysts, the reaction pressure was moved up to 0.7 MPa. Table 7 shows the results of experiments carried out on three chosen as representative catalysts at their optimal temperatures and at two values of reaction pressure: 0.1 and 0.7 MPa.
Table 7. Activity and selectivity of catalysts at optimal temperatures (Topt) and at different pressures (P).
| Catalysts | P (MPa) | Topt (°C) | X (%) | Si−C6 (%) | Yi−C6 (%) | 2,3-DMB:2-MP:3-MP | Scr (%) | RON | Lifetime (h) |
|---|---|---|---|---|---|---|---|---|---|
| Pd/HY | 0.1 | 350 | 32 | 59 | 19 | 1:12:7 | 37 | 30 | 1.25 |
| 0.7 | 300 | 82 | 85 | 66 | 1:3:1.7 | 7 | 57 | 14 | |
| Pd/Al-HY(2.5:1) | 0.1 | 325 | 38 | 94 | 36 | 1:12:6 | 6 | 56 | 23.7 |
| 0.7 | 325 | 82 | 81 | 70 | 1:3:2 | 4 | 60 | > 34 | |
| Pd/HZSM-5–500 | 0.1 | 275 | 66 | 76 | 50 | 1:23:12 | 24 | 58.5 | 1.0 |
| 0.7 | 250 | 79 | 98 | 77 | 1:59: 34 | 2 | 65.5 | > 30 |
As seen in table 7, at 0.7 MPa, all three catalysts gave higher values of conversion, selectivity and isohexane yield than those obtained at atmospheric pressure. On catalysts Pd/HY and Pd/HZSM-5-500 the optimal reaction temperature even decreased 50 and 25 °C, correspondingly. Also at pressure of 0.7 MPa one can observe remarkable reductions in cracking selectivity of all the catalysts and herewith significant improvements of their lifetimes. RON values of liquid products, obtained at 0.7 MPa on all the catalysts were higher compared to the case when the reaction proceeded at atmospheric pressure. Thus, assuming all experimental results one can conclude that among the studied catalysts, sample 0.8 wt% Pd/HZSM-5-500 has been shown to have the best activity, selectivity and stability in n-hexane isomerization at 0.7 MPa. The only drawback of this catalyst is low proportion of two-branched isomers in reaction products.
4. Conclusions
Calcination temperature of (NH4)ZSM-5 affected physico- chemical properties and activity of the obtained catalysts; optimal calcination temperature is 500–550 °C.
Compared to catalyst Pd/HY, catalyst Pd/HZSM-5 is characterized by smaller Pd cluster, higher metal dispersity, reduction extent and acidity, therefore its activity in isohexane formation has been found higher, but on this catalyst the proportion of two-branched isomers was lower, cracking selectivity higher and low stability at atmospheric pressure.
Addition (mixing) of aluminum oxide to zeolite reduced the acidity of catalyst which led to decrease of cracking selectivity and increase of catalyst stability. It is important to notice that if alumina addition improved physico-chemical properties of Pd catalysts supported on HY zeolites towards states being favorable for isomerization reaction, this addition affected the properties of Pd catalysts supported on HZSM-5 zeolites in the opposite direction. It has been indicated that for catalysts Pd/Al-HY the optimal composition ratio in carrier is Al2O3:HY = 2.5:1.
Increasing reaction pressure from 0.1 to 0.7 MPa resulted in remarkable increase in activity, selectivity and stability of catalysts. At 0.1 MPa, catalyst Pd/Al-HY(2.5:1) expressed the highest stability, but at 0.7 MPa, catalyst Pd/HZSM-5-500 has been found to be the best catalyst.
Acknowledgment
The research group acknowledges the financial support from the Materials Science Council, Vietnam Academy of Sciences and Technology.