Abstract
A simple and eco-friendly method has been developed for the synthesis of gold nanoparticles using allamanda flower extract. In this green synthesis method, chloroauric acid (HAuCl4) solution was reduced with the help of allamanda flower extract. The synthesized gold nanoparticles were characterized by atomic force microscopy (AFM), transmission electron microscopy (TEM) and x-ray diffraction technique for their morphological and structural analysis. The size of the spherical and triangular gold nanoparticles was found to be in the range of 5–40 and 20–70 nm, respectively. The x-ray diffraction analysis revealed that the crystallite size of face-centered cubic (FCC) gold nanoparticles was ∼ 11 nm. These synthesized gold nanoparticles exhibit good catalytic activity towards the reduction of H2O2. The fabricated sensor exhibits good sensitivity of 21.33 μA mM−1 cm−2 with linear relationship (R2 = 0.996) in the range from 2 to 10 mM of H2O2 concentration. This work can be extended further for potential applications such as antimicrobial studies, bio-imaging and drug-delivery owing to the known properties of the allamanda flower extract.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, metal nanoparticles have been an intense topic of research due to their size, shape, surrounding media and inter-particle distance dependent physical, chemical, optical, electronic and electrical properties [1–6]. In particular, gold nanoparticles have attracted the attention of researchers because of their unique optical properties originating from surface plasmon resonance (SPR) [7]. There are various methods for the synthesis of gold nanoparticles such as wet chemical [8], biological [9], laser ablation [10], electrochemical [11], etc. In chemical methods, various precursors, reducing agents and capping agents are used which could be hazardous for human beings and the environment. To overcome these problems, researchers are using bacteria [12], yeast [13] and plant/flower extracts [14, 15] for the synthesis of nanoparticles, which is called green synthesis/biosynthesis. The green synthesis has drawn attention from researchers because of its rapid, clean, nontoxic, economical, eco-friendly procedure [16,17]. Nanoparticles synthesized by this technique show the same properties as those synthesized by any other technique such as wet chemical, laser ablation and electrochemical.
For the biosynthesis of gold nanoparticles, researchers have reported various plants, leaves, fruits and flower extracts such as lemongrass [18], coriander [19], neem [20], aloe vera [21], rosa rugosa [22], cypress leaves [23], barbated skull cupherb [24], tansy fruit [25], magnolia kobus and diopyros kaki [26]. Recently, Maity et al [27] have synthesized gold nanoparticles using gum polysaccharide of cochlospermum religiosum (katira gum), and studied their role as a catalyst in the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). Ghoreishi et al [28] have also reported green synthesis of silver, gold nanoparticles using rosa damascena and their primary applications in electrochemistry.
The present paper delineates biosynthesis of gold nanoparticles by allamanda flower extract. To the best of our knowledge, this is the first report that describes the biosynthesis of gold nanoparticles by allamanda flower extract. Allamanda also known as golden trumpet exhibits various medicinal properties. The plant is used against jaundice, malaria and enlarged spleen. The allamanda flower acts as a laxative and also shows antibiotic properties [29]. Synthesized gold nanoparticles were found to be anisotropic with the size of the spherical and triangular gold nanoparticles to be in the range of 5–40 and 20–70 nm, respectively. The x-ray diffraction (XRD) analysis revealed that the crystallite size of FCC gold nanoparticles was ∼ 11 nm. The nanoparticles further showed good catalytic activity towards the reduction of H2O2.
2. Experimental
Gold chloride (HAuCl4·3H2O), potassium dihydrogen ortho phosphate (KH2PO4), sodium hydroxide (NaOH) and hydrogen peroxide (H2O2 30%) were purchased from Thomas Baker, India. All chemicals were used as received without further purification. The allamanda cathartica (family, apocynaceae) flowers were collected from the garden at DIAT campus. Deionized water (DI water, ≈ 18 MΩ) was used in the experiment.
Twenty-five allamanda flowers (∼ 20 g) were washed and then boiled in 100 ml of DI water for 2 h. Flower extract was filtered thrice and stored at 4 °C for further experiment. The color of the filtered flower extract (60 ml) was found to be a faint yellow. For the synthesis of gold nanoparticles, 20 ml of 1 mM gold chloride was stirred at 50 °C for 10 min in three different flasks simultaneously. A quantity of 5, 7.5 and 10 ml of flower extract was added rapidly into the three different precursor solutions. All these samples were labeled as R1, R2 and R3, respectively. Within 10 min, the color of the solution of sample R1 and R2 changed from pale yellow to purplish red indicating the formation of gold nanoparticles. The color of sample R3 changed from pale yellow to blue indicating some kind of agglomeration. In order to ensure the completion of the reaction, the solution was continuously stirred for another 30 min but no color change was observed. The synthesized gold nanoparticles were stable for several weeks.
Synthesized gold nanoparticles were examined under UV–Vis spectroscopy (Ocean Optics, HR 4000), Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer), atomic force microscopy (AFM, Asylum Research) and transmission electron microscopy (TEM, FEI-Tecnai G2 20). For TEM characterization, highly diluted samples were prepared and directly deposited on carbon coated copper grid. These samples were dried under an IR lamp. The XRD samples were prepared by adding several drops of the gold nanoparticles solution on cleaned glass substrate followed by drying.
For the electrochemical studies, glassy carbon electrodes (GCEs) were polished with 1.0, 0.3 and 0.05 μm alumina powder and rinsed with ethanol and DI water successively. These electrodes were dried in natural environment and modified by casting the 10 μl drop of synthesized Au nanoparticles. Further, a 1.8 μl drop of polyaniline (PANI) 0.8% was also casted onto the GCE and kept in an oven for 12 h at 60 °C. Similarly for reference, gold nanoparticles and PANI modified electrodes were also prepared. All electrochemical experiments were performed with electrochemical workstation (CHI 1100 B), three-electrode cell, in 0.1 M phosphate buffer solution (PBS) at 7.4 pH. Ag/AgCl and platinum wire electrodes were used as reference and counter electrodes, respectively, to measure the potentials.
3. Results and discussion
Figure 1(a) shows UV–Vis spectra of allamanda flower extract while the inset shows the yellow flower and its extract. The UV–Vis spectra show a strong absorption peak at ∼370 nm. Figure 1(b) shows UV–Vis spectra of gold nanoparticles synthesized at different volumes of flower extract i.e. R1 = 5.0 ml, R2 = 7.5 ml and R3 = 10 ml. It can be observed from the spectra that at low volume (R1) of flower extract an absorption band appears at ∼ 552 nm which is a characteristic peak of gold nanoparticles due to surface plasmon resonance. When the volume of flower extract was increased to R2, a small absorption band appears at ∼ 715 nm along with the characteristic band of Au nanoparticles at ∼ 545 nm. These two distinct absorption bands indicate asymmetry in the structure of gold nanoparticles and may be associated with the transverse and longitudinal plasmon absorption. At higher volume of flower extract (R3), a broad absorption band appeared at ∼ 590 nm indicating aggregation of gold nanoparticles within the solution. Samples R1 and R2 were used for further characterization and catalytic application.
Figure 1. (a) UV–Vis spectra of allamanda flower extract while the inset shows the flower and its extract, (b) UV–Vis spectra of gold nanoparticles synthesized at different volumes of flower extract i.e. R1 = 5.0, R2 = 7.5 and R3 = 10 ml.
Download figure:
Standard image High-resolution imageFigure 2 shows the FTIR spectra for (i) aqueous extract of allamanda flower and (ii) synthesized gold nanoparticles. Here spectra (i) show intense peaks at 3347 cm−1 ( stretch), 2337 cm−1 (
stretch), 2337 cm−1 ( stretch), 1639 cm−1 (
stretch), 1639 cm−1 ( stretch), 1071 cm−1 (
stretch), 1071 cm−1 ( stretch) and 663 cm−1 (
stretch) and 663 cm−1 ( stretch). All these peaks in FTIR spectra confirm various components of allamanda floral extract [30]. Spectra (ii) also shows all these intense peaks with shift in
stretch). All these peaks in FTIR spectra confirm various components of allamanda floral extract [30]. Spectra (ii) also shows all these intense peaks with shift in  stretching from 3347 to 3306 cm−1 and
stretching from 3347 to 3306 cm−1 and  stretching from 1639 to 1649 cm−1. These shifts may be attributed to the reduction of Au3+ to Au0 and capping of synthesized gold nanoparticles.
stretching from 1639 to 1649 cm−1. These shifts may be attributed to the reduction of Au3+ to Au0 and capping of synthesized gold nanoparticles.
Figure 2. FTIR spectra for (i) aqueous extract of allamanda flower and (ii) synthesized gold nanoparticles.
Download figure:
Standard image High-resolution imageFigure 3 shows the XRD spectra of sample R2. The XRD pattern exhibits sharp reflections which are characteristics of face-centered cubic (FCC) gold. The spectra shows four characteristic peaks of gold at 38.28°, 44.44°, 64.77° and 77.72° in the 2θ range of 20–80° which are indexed as (111), (200), (220) and (311) planes (JCPDS file no. 04-0784). The full-width at half-maximum (FWHM) of (111) diffraction plane was used to estimate the average crystallites size of gold nanoparticles by the Scherrer formula. The calculated crystallites size was found to be ∼11 nm. The average of lattice parameter 'a' corresponding to all characteristic peaks was calculated and found to be 4.06897 Å in close agreement with the standard value 4.078 Å (JCPDS file no. 04-0784). Inter-planer spacings corresponding to (111), (200), (220) and (311) planes were also determined and found to be 2.348, 2.036, 1.437 and 1.227 Å, respectively.
Figure 3. XRD of synthesized gold nanoparticles by flower extract (R2).
Download figure:
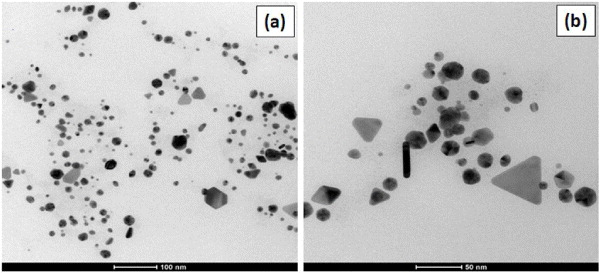
Standard image High-resolution imageFigure 4(a) shows the AFM image of synthesized nanoparticles in non-contact mode. The image clearly shows particles of different sizes ranging from 5 to 100 nm. Figure 4(b) shows single line profile of the nanoparticles as shown in figure 4(a). AFM images, though not clear, show that the particles are of different shapes. Since the XRD result shows intense presence of 111 plane, we expected to observe anisotropic structures along with the spherical nanoparticles and this was verified using TEM analysis (figure 5). It can be observed that the anisotropic shapes of gold nanoparticles such as spherical, triangular, pentagonal and hexagonal have been synthesized. Spherical gold nanoparticles were found in the majority as compared to other shapes. The size of the spherical gold nanoparticles was observed to be in the range of 5–40 nm. Triangular gold nanoparticles were found to be in the range of 20–70 nm. The TEM results are also supporting the UV–Vis spectroscopic result, which shows anisotropic nanostructures of gold.
Figure 4. (a) AFM image of synthesized gold nanoparticles by flower extract (R2), (b) single line profile of synthesized nanoparticles as shown in (a).
Download figure:
Standard image High-resolution imageFigure 5. TEM images of synthesized gold nanoparticles by flower extract (R2).
Download figure:
Standard image High-resolution imageThe cyclic voltammograms of bare, PANI, gold NPs and gold NPs + PANI modified GC electrodes in air saturated PBS solution (pH 7.4) in the presence of 2 mM H2O2 are shown in figure 6(a). It can be observed from this figure that bare, PANI and gold NPs modified GC electrodes do not exhibit noticeable electro-catalytic response towards H2O2. In contrast, gold NPs + PANI modified GC electrode shows high electro-catalytic activity towards reduction of H2O2. Figure 6(b) shows cyclic voltammograms of gold NPs + PANI modified GC electrode in air saturated PBS solution (pH 7.4) in the presence of various concentrations of H2O2. It can be seen that a strong reduction current peak appears at +0.023 V, which may be attributed to the electrochemical reduction of H2O2 and O2. It is also found that the reduction current at positive potential increases with the increased amount of H2O2 concentration. A strong oxidation current peak is also observed at −0.26 V and may be attributed to the oxidation of H2O2 in presence of O2. The inset shows the calibration curve between the anodic peak currents and H2O2 concentrations. A good linear relationship (R2 = 0.996) with sensitivity 21.33 μA mM−1 cm−2 has been observed in the ranges from 2 to 10 mM.
Figure 6. (a) Cyclic voltammograms of bare, PANI, gold NPs and gold NPs + PANI modified GC electrodes in air saturated PBS solution (pH 7.4) in the presence of 2 mM H2O2, (b) cyclic voltammograms of gold NPs + PANI modified GC electrode in air saturated PBS solution (pH 7.4) in the presence of various concentrations of H2O2. Inset shows the corresponding calibration curve (R2 = 0.996) between the anodic peak current and H2O2 concentrations.
Download figure:
Standard image High-resolution image4. Conclusions
Gold nanoparticles have been synthesized with the help of allamanda flower extract. The TEM analysis reveals that the size of the spherical, triangular gold nanoparticles is in the ranges of 5–40 and 20–70 nm respectively. The XRD analysis indicates that the crystallite size of gold nanoparticles is ∼ 11 nm with FCC structure. The synthesized gold nanoparticles demonstrate good electro-catalytic activity towards the reduction of H2O2. The sensor shows linear response (R2 = 0.996) to H2O2 in the ranges from 2 to 10 mM with sensitivity 21.33 μA mM−1 cm−2. The nanoparticles synthesized from allamanda flower extract do not require any capping agent and remain stable for several weeks. The extract acts as a capping agent coating the particle and restricting further growth of the particles. This opens up very useful applications for such nanoparticles, as the allamanda flower is known to have potential medicinal properties. This work can be extended for potential applications such as antimicrobial studies, bio-imaging and drug-delivery.
Acknowledgments
The authors thank Dr Prahlada, Vice Chancellor, Defence Institute of Advanced Technology (DIAT), Deemed University, Pune for providing the laboratory facilities and financial assistance. SSD acknowledges funding from Defence Research and Development Organization-Defence Institute of Advanced Technology (DRDO-DIAT) Program on Nanomaterials by Extramural Research & Intellectual Property Rights ER&IPR, DRDO.