Abstract
The present work reports the use and application of a novel protic ionic liquid (triethylammonium bis(trifluoromethylsulfonyl)imide; NEt3H TFSI) as an electrolyte for symmetric planar micro-supercapacitors based on silicon nanowire electrodes. The excellent performance of the device has been successfully demonstrated using cyclic voltammetry, galvanostatic charge-discharge cycles and electrochemical impedance spectroscopy. The electrochemical characterization of this system exhibits a wide operative voltage of 4 V as well as an outstanding long cycling stability after millions of galvanostatic cycles at a high current density of 2 mA cm−2. In addition, the electrochemical double layer micro-supercapacitor was able to deliver a high power density of 4 mW cm−2 in a very short time pulses (a few ms). Our results could be of interest to develop prospective on-chip micro-supercapacitors using protic ionic liquids as electrolytes with high performance in terms of power and energy densities.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Recently, micro-supercapacitors, also known as micro-ultracapacitors, have emerged as alternative and prospective electrochemical energy storage devices due to their interesting properties in terms of high power density, efficiency, fast charge and discharge rate, excellent reversibility, long lifespan and relatively low cost, which make them very attractive as micro-power sources for different technological applications [1]. Over the past years, these kinds of devices have attracted considerable attention owing to their possible integration into miniaturized, wearable and portable electronics devices such as micro-mechanical systems, micro-robots, smartcards, radio frequency identification (RFID) tag or medical micro-devices (e.g. implants) [2, 3]. The rapid growth and demand of these technological applications has triggered an important advancement in the scientific research field of novel nano-materials and electrolytes in order to provide ultra-high performance micro-supercapacitors in terms of power and energy densities [4, 5]. Within this context, nanostructured silicon such as silicon nanowires (SiNWs) [6–10], silicon nanotrees (SiNTrs) (e.g. hyperbranched SiNWs) [11] or silicon carbide nanowires (SiCNWs) [12–15] has sparked a great deal of interest as nanoscale materials for micro-supercapacitor devices due to their high surface specific area, high power pulse and long cyclability.
In parallel, during the last decade tremendous efforts have also been devoted to study the effect of electrolyte in different energy technological applications such as for example batteries, fuel and solar cells, actuators or micro-supercapacitors [16, 17]. In this way, ionic liquids (ILs) and derivatives (e.g. ionogels) have been selected as one of the most novel and advanced electrolytes in the performance of energy storage devices (e.g. micro-supercapacitors) due to their interesting properties regarding their high thermal stability (>300 °C), wide electrochemical window (>4 V), non-flammability and very low vapor pressure [18–20]. Accordingly, one of the most important characteristics in the performance of supercapacitors is related with their specific energy (E = 1/2CV2), where C is the capacitance and V is the cell voltage and the maximal power density (P = V2/4ESR), where ESR is the equivalent series resistance. As can be seen, both properties, energy and power densities, are proportional to V2. Typically, the cell voltage is mainly limited by the electrochemical stability of the electrolyte. According to these characteristics, ILs as electrolyte can play a key role on the performance of micro-supercapacitors since they can widen the cell voltage and consequently the properties related with power and energy densities could be increased. Currently, we have demonstrated the excellent performance of an aprotic ionic liquid (APIL) (N-methyl-N-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide; PYR13TFSI) as electrolyte in a micro-supercapacitor device based on SiNWs electrodes [6]. The results reflected an operating cell voltage of 4 V, a high maximal power density value of 182 mW cm−2 as well as an excellent electrochemical stability after millions of galvanostatic charge-discharge cycles (e.g. 25% of the initial capacitance was found to be lost) [6]. The use of this IL (PYR13TFSI) as an electrolyte in SiNWs-based micro-supercapacitors was proven as important progress regarding the values reported in the literature employing as an electrolyte a PC solution containing 1 M tetraethylammonium tetrafluoroborate (NEt4BF4) [21, 22]. Moreover, pyrrolidinium-based APILs have shown to be excellent electrolytes for supercapacitor operating at high temperatures [23]. Among ionic liquids protic ionic liquids (PILs), produced through proton transfer from a Bronsted acid to a Bronsted base, have aroused special attention as electrolytes in the field of supercapacitors (e.g. electrochemical double layer capacitors, EDLCs) and fuel cells due to their interesting physical-chemical properties in terms of higher conductivity, proton activity, fluidity as well as lower melting points by comparison with APILs [24, 25]. Nowadays, EDLCs made of active carbon electrodes in presence of several PILs based on phosphonium, ammonium or pyrrolidinium derivatives have already shown an excellent capacitive behaviour, a high cycling stability as well as a wide cell voltage [26–31]. Interestingly, over the past years a novel protic ionic liquid known as triethylammonium bis(trifluoromethylsulfonyl)imide (NEt3H TFSI) has been reported as an excellent electrolyte for carbon-based EDLCs [32–34]. However, to the best of our knowledge the performance of PILs has not been yet reported as electrolyte for micro-supercapacitors based on nanostructured materials.
Herein, we describe the performance of a symmetric planar micro-supercapacitor based on nanostructured silicon electrodes (e.g. SiNWs grown by chemical vapor deposition) using a NEt3H TFSI (scheme
Scheme 1. Chemical structure of triethylammonium bis(trifluoromethylsulfonyl)imide (NEt3H TFSI).
Download figure:
Standard image High-resolution image2. Experimental methodology
2.1. Materials and reagents
Highly n-doped Si (111) substrates (doping level: 5 × 1018 doping atoms cm−3) and resistivity less than 0.005 Ω cm were used as the substrate for SiNW growth. Gold colloid solution (50 nm) was purchased from British BioCell. Triethylammonium bis(trifluoromethylsulfonyl)imide (NEt3H TFSI) was purchased from IOLITEC (ionic liquids technologies GmbH, Germany) and used as received without further purification.
2.2. Growth of SiNWs
SiNWs electrodes with a length of approximately 35 μm and a diameter of 50 nm were grown in a CVD reactor (EasyTube3000 First Nano, a Division of CVD Equipment Corporation) by using the vapor-liquid-solid (VLS) method via gold catalysis on highly doped n-Si (111) substrate. Gold colloids with size of 50 nm were used as catalysts, H2 as carrier gas, silane (SiH4) as silicon precursor, phosphine (PH3) as n-doping gas and HCl as additive gas. SiNWs were grown using a standard deposition process reported in our previous works [6, 7]. The growth was performed at 600 °C, under 6 Torr total pressure, with 40 sccm (standard cubic centimeters) of SiH4, 100 sccm of PH3 gas (0.2% PH3 in H2), 100 sccm of HCl gas and 700 sccm of H2 as supporting gas.
2.3. Design of the micro-supercapacitor
Symmetric micro-supercapacitors were designed from nanostructured electrodes made of SiNWs (L = 35 μm, ϕ = 50 nm) with a geometric surface of 1 cm2. A homemade two-electrode supercapacitor cell was built by assembling two nanostructured electrodes separated by a Whatman glass fiber paper separator soaked with the electrolyte NEt3H TFSI.
2.4. Electrochemical characterization of micro-supercapacitors
Cyclic voltammetry (CV) curves and galvanostatic charge–discharge cycles were performed between 0 and 4 V using different scan rates (0.2–20 V s−1) and current densities (0.1–2 mA cm−2), respectively. Electrochemical impedance spectroscopy (EIS) measurements were performed using a sinusoidal signal of ±10 mV amplitude and a frequency range from 400 kHz to 10 mHz. The electrochemical stability of the device was obtained by performing galvanostatic charge-discharge cycles over 5 × 106 cycles at a current density of 2 mA cm−2 in the potential range from 0 to 4 V. Electrochemical tests were performed using a multichannel VMP3 potentiostat/galvanostat with Ec-Lab software (Biologic, France). All measurements were carried out using NEt3H TFSI as an electrolyte in an argon-filled glove box with oxygen and water levels less than 1 ppm at room temperature.
2.5. Morphological characterization
The morphology of the resulting SiNWs was examined by using a ZEISS Ultra 55 scanning electron microscope operating at an accelerating voltage of 10 kV.
3. Results and discussion
Figure 1(a) shows the morphology of the resulting SiNWs grown by the chemical vapor deposition process. The density of nanowires was calculated to be ∼108 nanowires per cm2 by counting the number of gold colloids as reported in our previous works [6, 10]. The overall length of the SiNWs was found to be approximately 35 μm as displayed in figure 1(b). The diameter of the nanowires was estimated to be 50 nm according to the colloid size employed in this study.
Figure 1. (a) SEM image of the surface morphology of SiNWs prepared by CVD via gold colloids recorded at 45° titled angle. (b) Cross sectional view of SiNWs.
Download figure:
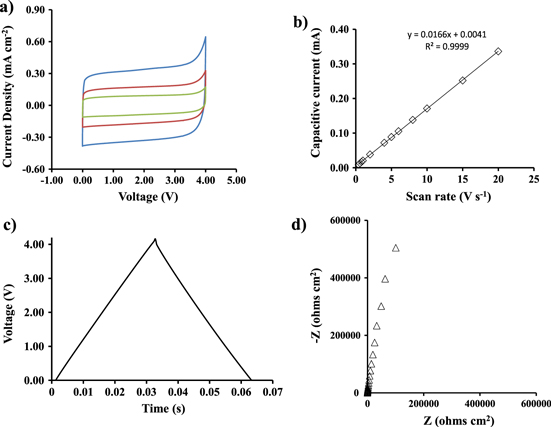
Standard image High-resolution imageFigure 2 shows the electrochemical characterization of the symmetric micro-supercapacitor at room temperature using NEt3H TFSI as electrolyte. As can be seen in figure 2(a), cyclic voltammograms reflect a nearly rectangular shape at different scan rates (5, 10 and 20 V s−1, respectively) demonstrating an excellent capacitive behavior and a high rate capability in a wide cell voltage range of 4 V. It is worth noting that the straight rectangular shape was kept even at high scan rates reflecting the excellent reversibility and faster ionic diffusion in the electrolyte–electrode interface. The enhancement of the voltage window up to 4 V and the excellent capacitive behavior at a high scan rate of 20 V s−1 represent an outstanding improvement compared with the conventional EDLCs based on carbon electrodes. A slightly distortion of the CV curves was attributed to the oxidation of silicon to SiO2 according to the increase of the current reflected in figure 2(a). This behaviour was also observed in systems based on SiNWs or SiCNWs using aqueous electrolytes (e.g. 1 or 3.5 M KCl) [8, 35] or organic solvents [10]. Thus, the silicon oxidation on SiNWs has been reported due to the presence of water traces [36]. Figure 2(b) shows a linear relation between the capacitive current and the scan rate, which demonstrates a negligible ohmic drop in the electrolyte bulk and no redox peaks between the electrodes and the electrolyte were observed. According to figure 2(b), a specific capacitance (SC) value of approximately 17 μF cm−2 was calculated for the SiNWs micro-supercapacitor using the CV curves. The value was estimated from the relation
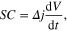
where Δj corresponds to the current density differences [Δj:(jox—jred)/2 expressed in mA cm−2] in the middle of the voltage window (2 V) and dV/dt is the scan rate (V s−1). This value was found higher than SiNWs-based micro-supercapacitors performed in a PC solution using a narrow electrochemical window of ∼1 V (e.g. 10 μF cm−2) [21]. Figure 2(c) displays the galvanostatic charge–discharge cycle of the device using a high current density of 2 mA cm−2. The profile shows a nearly symmetric triangular shape and fairly linear slopes between 0 and 4 V reflecting once again the excellent capacitive behavior. The specific capacitance of the micro-supercapacitor device was obtained from the equation
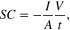
where the I is the applied current, A is the area of the electrode and V/t corresponds to the slope of discharging curve. According to this equation, a specific capacitance value was determined to be about 16 μF cm−2 which was found to be in excellent agreement with the CV curves. This value was found to be constant at current densities higher than 0.25 mA cm−2. Additionally, the device was able to be charged and discharged in a very short time pulse (e.g. ∼0.03 s for the discharge time at a current density of 2 mA cm−2). Figure 2(d) corresponds to the Nyquist plot of the device in a frequency range from 400 kHz to 10 mHz at 0 V. The impedance spectra of SiNWs micro-supercapacitors reflects a straight line in the low frequency range. The vertical profile at low frequencies indicates pure capacitive behavior, which is associated with the good diffusion of ions into the structure. EIS is also a powerful tool in order to determine some other important parameters in the performance of micro-supercapacitors such as the ESR or time constant of the device (τ0). Precisely, τ0 is defined as the minimum time needed to discharge all the energy from the device with an efficiency of more than 50% [37]. This parameter was calculated using the relation (τ0 = 1/f0) according to a previous work reported by Taberna et al [38]. This value was found to be 4.6 ms, which was found to be significantly lower than other micro-supercapacitors reported in the literature based on carbon electrodes (e.g. values ranging from 26 to 700 ms) [1]. Thus, SiNWs-based micro-supercapacitors show a great potential for the instantaneous delivery of high power. Accordingly, a power density (P) value of 4 mW cm−2 was obtained from the galvanostatic charge–discharge cycle displayed in figure 2(c). The power density was estimated from the relation P = E/t where E (expressed in mJ cm−2) is the specific energy which was described in the introduction and t (in s) is the discharge time. This value was found to be higher than another planar micro-supercapacitors based on carbon derivatives such as graphene (P: 9 μW cm−2) or carbon nanotubes (P: 0.28 mW cm−2) [1], SiCNWs [13] or SiNWs performed in organic solvents [21]. Overall, the electrochemical response of the device using the CV curves, Nyquist plot and galvanostatic charge–discharge cycles reflect two important features compared with carbon-based EDLCs: (1) the enlargement of the electrochemical window up to 4 V with a quasi-pure capacitive behavior at very high scan rates, and (2) the ultra-fast capability of the device to be charged and discharged able to deliver high power density values. These results demonstrate the excellent capacitive performance of SiNWs in a 2-electrode configuration in the presence of NEt3H TFSI. It is worth noting that a similar tendency was also observed for SiNWs-based micro-supercapacitors using APILs as electrolyte (e.g. PYR13TFSI) [6]. In conclusion, ILs can be employed as promising electrolytes in energy storage micro-devices as for example micro-supercapacitors based on SiNWs in the near future.
Figure 2. Characterization of a SiNWs-based micro-supercapacitor using NEt3H TFSI as electrolyte at room temperature. (a) Cyclic voltammograms at different scan rates: 20 V s−1 (blue line), 10 V s−1 (red line) and 5 V s−1 (green line). (b) Evolution of the capacitive current versus scan rate. (c) Galvanostatic charge-discharge cycles at a current density of 2 mA cm−2 between 0 and 4 V. (d) Nyquist plots measured at a voltage of 0 V. The impedance was measured using a frequency range from 400 kHz to 10 mHz.
Download figure:
Standard image High-resolution imageIn this work, the long cycle life of the device was evaluated by applying a million charge–discharge galvanostatic cycles at a high current density of 2 mA cm2 between 0 and 4 V. The percent of retained capacitance as a function of the cycle number is shown in figure 3(a). After 5 × 106 charge–discharge cycles, only ∼27% of the initial capacitance was found to be lost. More importantly, most of the degradation of the initial performance of the device occurs during the first 700 000 cycles according to the silicon oxidation, after which the system showed stabilization, reaching a plateau-like profile. This loss of specific capacitance at the beginning of the cycling test was observed in our previous work using SiNWs in a 3-electrode configuration in the presence of an aprotic ionic liquid (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIM TFSI)) [39] as electrolyte or in a 2-electrode configuration using PYR13TFSI as electrolyte [6]. Regarding this issue, this phenomenon was attributed to the complete oxidation of the topmost and at least first underlying atom layer of silicon [39]. After galvanostatic cycling, CV curve showed a pure rectangular shape even after several million galvanostatic charge-discharge cycle, as displayed in figure 3(b). Accordingly, the oxidation peak identified at the beginning of the cycling (figure 1(a)) was suppressed after cycling indicating passivation of the surface [8]. As can be illustrated in figure 3(b), the device exhibits a pure and excellent capacitive behaviour demonstrating its long and remarkable stability at a wide cell voltage of 4 V.
Figure 3. (a) Lifetime testing of the SiNWs micro-supercapacitors performed using 5 × 106 complete charge-discharge cycles at a current density of 2 mA cm−2 between 0 and 4 V. (b) Cyclic voltammogram after complete galvanostatic cycling at a scan rate of 20 V s−1.
Download figure:
Standard image High-resolution image4. Conclusions
This manuscript reports the application of a PIL (NEt3H TFSI) as electrolyte for planar micro-supercapacitors based on SiNWs. The performance of the electrochemical device shows an excellent capacitive behaviour in a wide cell voltage as high as 4 V. In addition, the micro-supercapacitor exhibits a high power density of 4 mW cm−2 and an outstanding electrochemical stability after several million galvanostatic charge-discharge cycles with a remarkable reversibility. The results of this investigation have been compared with previous works using aprotic ionic liquids as electrolytes. The performance of both APILs and PILs demonstrates that they can be employed as alternative electrolytes for electrochemical micro-supercapacitors with similar capacitive properties. Hence, novel ultra-high performance micro-supercapacitors could be designed and integrated in miniaturized electronic devices using ILs as electrolytes. To conclude, the performance of SiNWs-based micro-supercapacitors in the presence of ILs as electrolytes represents an innovative breakthrough in the field of conventional carbon-based EDLCs.
Acknowledgments
The authors acknowledge the CEA for financial support of this work. This NEST project has received funding from the European Union's Seventh Programme for research, technological development and demonstration under grant agreement No 309143 (2012–2015).
Footnotes
- *
Invited talk at the 2nd International Workshop on Nano Materials for Energy Conversion NMEC-2, 17–20 November, 2014, Ho Chi Minh City, Vietnam.