Abstract
Chemical modification of silicon nitride (SiN) surfaces by silanization has been widely studied especially with 3-(aminopropyl)triethoxysilane (APTES) and 3-(glycidyloxypropyl) dimethylethoxysilane (GOPES). However few reports performed the experimental and computational studies together. In this study, surface modification of SiN surfaces with GOPES and APTES covalently bound with glutaraldehyde (GTA) was investigated for antibody immobilization. The monoclonal anti-cytokeratin-FITC (MACF) antibody was immobilized on the modified SiN surfaces. The modified surfaces were characterized by water contact angle measurements, atomic force microscopy and fluorescence microscopy. The FITC-fluorescent label indicated the existence of MACF antibody on the SiN surfaces and the efficiency of the silanization reaction. Absorption of APTES and GOPES on the oxidized SiN surfaces was computationally modeled and calculated by Materials Studio software. The computational and experimental results showed that modification of the SiN surfaces with APTES and GTA was more effective than the modification with GOPES.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Silicon nitride (SiN) is an important material in micro and nano devices especially biosensors; however, studies on SiN surface modification are still limited [1–5]. Recently, chemical modification of silicon and silicon derivation surfaces by silanization has been widely studied [3, 5–26]. Many silane compounds have been used such as amino organosilane (3-(aminopropyl) triethoxysilane (APTES), 3-(aminopropyl) trimethoxysilane, p-(aminophenyl) trimethoxysilane, 3-(aminopropyl) tris(methoxyethoxyethoxy)silane, 3-(aminopropyl) dimethylethoxysilane), thiol organosilane (3-(mercaptopropyl) triethoxysilane), epoxy organosilane (3-(glycidoxypropyl) trimethoxysilane (GOPTMS), 3-(glycidoxypropyl) triethoxysilane (GOPTES), 3-(glycidoxypropyl) dimethylethoxysilane (GOPES)), aldehyde organosilane (triethoxysilylundecanal, triethoxsilylbutyraldehyde), vinyl organosilane (vinyltriethoxysilane, vinyltrimethoxysilane), carboxylate, phosphonate and sulfonate organosilane etc [7, 26, 27]. Among of them, APTES is the most popular and it has been used in modification of silicon [12, 15–18, 20–23, 25] and other substrates [19, 28] but little on SiN substrates [24]. GOPES has also been used to modify silicon surfaces [8–10] and rarely on SiN surface either [11].
The immobilization of bio-receptor on silica surfaces with amino and epoxy functional modification showed that amino group is more effective than epoxy group. Pijanowska et al [13] modified silica surfaces with GOPTES and APTES covalent with glutaraldehyde (GTA) for immobilization of enzyme urease. The surface modification was characterized by atomic force microscopy (AFM), time-of-flight secondary ion mass spectroscopy (ToF-SIMS) and fourier transforms infrared spectroscopy. The results showed that the amount of protein deposited on the surface modified with APTES was greater than with GOPTES. Awsiuk et al [5] also modified SiN surfaces with APTES and GOPTMS for immobilization of rabbit gamma globulins (rIgG) and bovine serum albumin. XPS, AFM and ToF-SIMS results also revealed that the content of antibody on the SiN surfaces modified with APTES was larger than with GOPTMS. These results imply that covalent binding of APTES on silica may be stronger than GOPTES and GOPTMS. It can be predicted that modification with APTES on SiN substrates may also be more effective than with GOPES.
Reports on silica surface modification rarely presented the experimental and computational studies together except a few studies on molecular structure of APTES covalently bound to silicon surfaces [12]. Silanization of silica substrates with GOPES has been studied by high-resolution electron energy loss spectroscopy and scanning tunneling microscopy and explained by simulation structure of GOPES in vertical and horizontal conformation [9].
In this study surface modification of SiN surfaces with GOPES and APTES covalently bound with GTA was investigated for antibody immobilization. AFM and water contact angle measurements were employed to characterize the modified SiN surfaces. Monoclonal anti-cytokeratin-FITC (MACF) antibody was labeled by fluorescent indicator (FITC) which can be observed by fluorescence microscopy to evaluate efficiency of APTES and GOPES immobilization. Absorption of APTES and GOPES on silica surfaces was simulated by Materials Studio software (version 5.0). The computational and experimental results showed that the modification of the SiN surfaces with APTES covalently bound with GTA was more effective than the modification with GOPES.
2. Experimental
2.1. Materials
GOPES (≥98%), APTES (≥98%), GTA (25% in H2O), phosphate buffered saline (PBS) pH 7.4 and MACF antibody were purchased from Sigma-Aldrich (USA). Acetone and absolute ethanol were purchased from Merck (USA). H2SO4 (96%), H2O2 (30%) and deionized (DI) water were from Chinese companies.
SiN samples cut from the silicon (100) wafers coated with 1000 nm SiN layer by LPCVD technique.
2.2. Experimental
2.2.1. Modification of the SiN surfaces with GOPES and APTES and immobilization of the MACF antibody
The SiN samples were washed and cleaned by soaking in acetone, ethanol and piranha solution (volume proportion of H2SO4 96%: H2O2 30% = 2:1) respectively and then rinsed with DI water and dried by N2 flow after each cleaning steps to eliminate stains. After that, the SiN surfaces were oxidized by the UV/ozone instrument (BioForce Nanosciences, Australia) for 10 min to generate the functional silanol Si–OH.
Subsequently, the oxidized SiN substrates were modified by two processes. In the first process, the SiN substrates were soaked in the GOPES 3% solution with H2SO4 1 M in water. Silanization reaction with GOPES was performed at 50 °C overnight and left free epoxy end chain on the SiN surface.
In the second process, the oxidized SiN substrates were soaked in the APTES 1% solution in ethanol for 3 h to form siloxane monolayers condensed on the SiN surface. Then the SiN substrates were incubated at 110 °C for 10 min after being rinsed with ethanol. After APTES treatment, the SiN substrates were soaked again with the GTA 2% solution in PBS for 2 h. Then the substrates were rinsed with PBS. GTA with two aldehyde groups is used to link the amine function on APTES end side and the amine function groups on antibody.
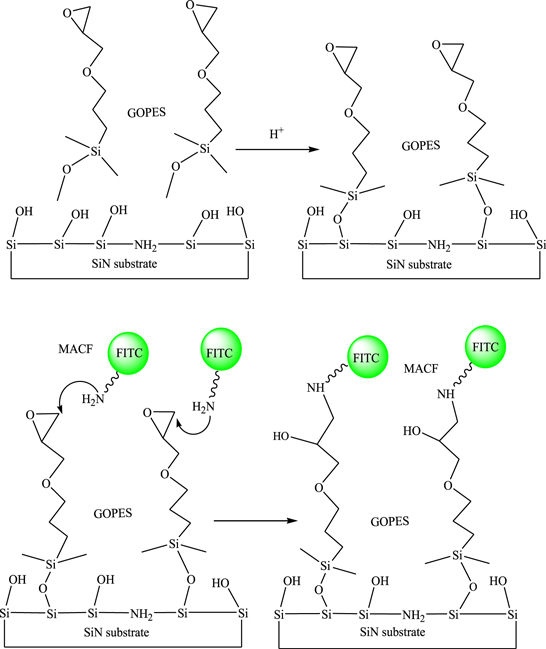
Finally, the modified SiN substrates were soaked in MACF antibody with concentration of 5 μg ml−1 in PBS solution overnight. Then the SiN substrates were rinsed with PBS and dried by N2 flow. The schemes of surface modification with GOPES and APTES and immobilization of MACF antibody are presented in figures 1 and 2.
Figure 1. Scheme of modification with GOPES and immobilization of MACF antibody on the SiN surface.
Download figure:
Standard image High-resolution imageFigure 2. Scheme of surface modification with APTES and GTA and immobilization of MACF antibody on the SiN surface.
Download figure:
Standard image High-resolution image2.2.2. Modeling and calculation of the absorption energy of APTES and GOPES molecules on silicon surface
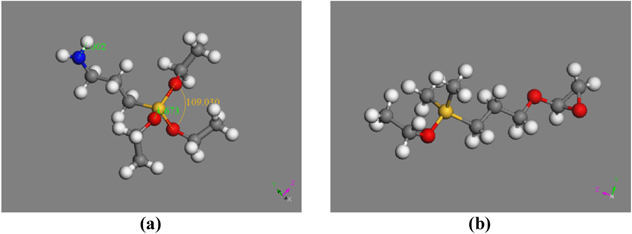
APTES and GOPES molecules were constructed by Sketch Atom tool of the Materials Studio software and optimized by Forcite calculation/Geometry Optimization with COMPASS Forcefield (figure 3).
Figure 3. APTES (a) and GOPES (b) molecules after geometry optimization (Carbon atoms are in grey, silicon atom is in yellow, oxygen atoms are in red, nitrogen atom is in blue and hydrogen atoms are in white).
Download figure:
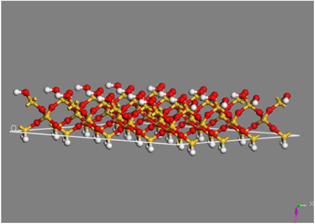
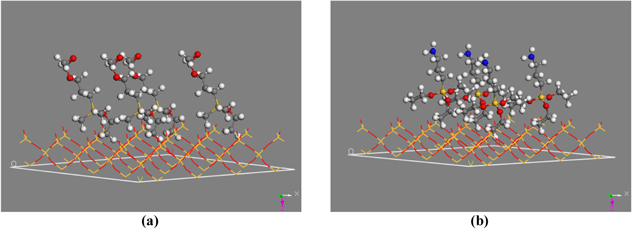
Standard image High-resolution imageSiO2 unit cell was extracted from the database by Import sample crystal SiO2 and multiplied 2 × 2 × 2 by supercell to create a SiO2 crystal. The SiO2 surface was made from (001) planar of the crystal by Cleave surface with a thickness of 5.47 Å. Finally, hydrogen was complemented to generate silanol groups Si–OH (figure 4). The absorption of APTES and GOPES molecules on the SiO2 surface was showed in figure 5 and the binding energy was calculated by DMol3/Energy tool.
Figure 4. A SiO2 surface made from (001) crystal planar. (Silicon atoms are in yellow, oxygen atoms are in red and hydrogen atoms are in white.)
Download figure:
Standard image High-resolution imageFigure 5. Absorption modeling of GOPES (a) and APTES (b) on the SiO2 surfaces. (Carbon atoms are in grey, silicon atoms are in yellow, oxygen atoms are in red, nitrogen atoms are in blue and hydrogen atoms are in white.)
Download figure:
Standard image High-resolution image2.3. Characterization
2.3.1. Water contact angle measurements
After each modification steps, the monolayer of silane and antibody deposited on surface lead to the change of surface wettability. The wetting properties of the modified surfaces were characterized by an optical drop profile analysis instrument CAM 101 (KSV Instruments Finland). The static water contact angle was measured many times, and average values were calculated.
2.3.2. Atomic force microscopy (AFM)
Images of the modified and un-modified surface were obtained by AFM characterization (Nanotec, Spain). Immobilization of molecules on the SiN surface leads to increase in surface roughness. The root-mean-square (RMS) roughness showed difference in the surface height in comparison with the average height in a topography image [1].
2.3.3. Fluorescent images
Thanks to fluorescent indicator FITC on the MACF antibody, fluorescent images of the modified SiN surfaces after antibody immobilization were obtained by fluorescence microscopy (Olympus BX41, Japan). This is the most observable method to evaluate modification efficiency of the SiN substrates.
3. Results and discussion
3.1. Water contact angle measurement
After each modification steps, the change in wetting property of the SiN surfaces was identified by static water contact angle. The results of the contact angle measurements are shown in table 1. The results showed that the wetting property of the modified SiN surfaces would be less hydrophilic after modification and immobilization in both cases of APTES and GOPES. Moreover, the modification of the SiN surfaces with APTES–GTA caused the SiN surfaces become more hydrophobic than with GOPES. The water contact angle of the SiN surface modified by APTES–GTA is always larger than GOPES. Deposition of more APTES molecules on the surface could cause the surface more hydrophobic than the modification with GOPES. It also could be due to epoxy functional groups of GOPES which are more hydrophilic leading to decrease in the contact angle value. The later hypothesis is less convinced than the former and it will be made clear in AFM analysis.
Table 1. Contact angle values of water of the SiN surfaces after each treatment step.
| Treatment step | Angle (°) with GOPES | Angle (°) with APTES–GTA |
|---|---|---|
| Before modification | 22.47 ± 0.87 | 22.47 ± 0.87 |
| After modification | 35.62 ± 2.14 | 45.63 ± 1.17 |
| After immobilization with MACF | 46.95 ± 1.35 | 50.84 ± 1.02 |
3.2. AFM characterization
Before modification, the SiN surfaces were cleaned by the piranha solution and oxidized by ozone. The RMS values were calculated as the average values of several measurements at different positions on the surface of the samples. The RMS of the SiN surfaces cleaned by piranha solution was RMS(p) = 0.94 ± 0.21 nm. The RMS of the SiN surfaces cleaned by UV ozone was RMS(o) = 1.11 ± 0.23 nm. The AFM results showed that the SiN surface after oxidized by ozone was rougher than by the piranha solution (figure 6).
Figure 6. AFM images and corresponding profiles of the SiN surfaces which were cleaned by (a) piranha solution and (b) UV ozone.
Download figure:
Standard image High-resolution imageAfter the APTES or GOPES modifications, the organic monolayer condensed on the SiN surface led to the smoother surface and decreased roughness of the modified surface. Figures 7(a) and (b) showed that the SiN surface after modification with GOPES had less roughness than the modification with APTES–GTA. It could imply that APTES–GTA was attached very well on the SiN surface. After the MACF antibody was immobilized, the growth of bio-molecules resulted in increasing roughness and hydrophobicity of the surface (figures 7(c) and (d)).
Figure 7. AFM images of the SiN surfaces after modification with (a) GOPES and (b) APTES–GTA, and after immobilization with MACF antibody with (c) GOPES and (d) APTES–GTA.
Download figure:
Standard image High-resolution imageThe change of roughness should be more visible via RMS values showed in table 2. The RMS of organic monolayer layers is usually from 1 to 2.5 nm [4].
Table 2. RMS roughness extracted from the AFM corresponding profiles of the SiN surfaces.
| With GOPES | With APTES–GTA | |
|---|---|---|
| RMS after piranha treatment (nm) | 0.94 ± 0.21 | 0.94 ± 0.21 |
| RMS after UV/ozone treatment (nm) | 1.10 ± 0.23 | 1.10 ± 0.23 |
| RMS after modification (nm) | 0.60 ± 0.09 | 0.82 ± 0.05 |
| RMS after immobilization with MACF (nm) | 1.89 ± 0.61 | 1.05 ± 0.21 |
The water contact angle of the SiN surface modified by APTES–GTA was larger than GOPES. It might be due to the deposition of more APTES molecules on the surface than GOPES. The RMS results showed that the surface roughness after modification with APTES was higher than with GOPES. It means that GOPES molecules might be less deposited on the surface causing low roughness. After MACF immobilization, the roughness increased dramatically due to the difference in areas with GOPES and without GOPES attachment on the surface. In contrast, the surface roughness after the APTES–GTA modification and antibody immobilization was not much different because the APTES molecules covered over the whole surface (table 2).
3.3. Fluorescent images
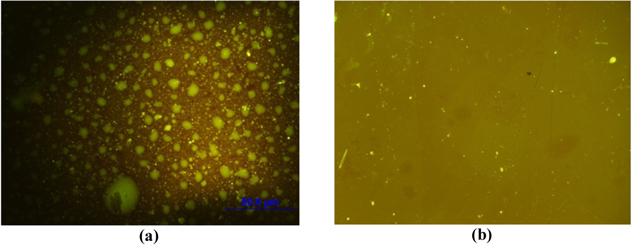
The good binding of APTES on the SiN surface is also supported by fluorescent images. The fluorescent images after immobilization of MACF antibody on the SiN substrates (figure 8) showed that the fluorescent light of the APTES modification was strong and continuous whereas the fluorescent light of the GOPES modification was weak and fragmentary. The strong linkage of the MACF antibody and the surface modified by APTES might come from the good affinity between the antibody and the functional groups on the modified agents and/or the good coverage of APTES leading to the good affinity with the antibody. The good coverage of APTES is assumed that three ethoxysilane groups of APTES could form crosslinked oligomer chains [12] resulting in a uniform layer deposited onto the SiN surface. In contrast, GOPES has one group of ethoxysilane which is really hard to form a continuous layer.
Figure 8. The fluorescent images of MACF immobilized on the modified SiN surfaces with GOPES (a) and APTES–GTA (b).
Download figure:
Standard image High-resolution image3.4. Computational results on binding energy
Table 3 shows the calculation results of binding energy of APTES and GOPES molecules attached to the silicon dioxide surface. The binding energies of GOPES and APTES with the silicon dioxide surface are approximately equal but total energy of APTES is lower than GOPES. It means that absorption of APTES on the surface is thermodynamically advantageous. It can also infer that covalent ability of APTES with the silicon dioxide surface is higher than GOPES.
Table 3. Calculation of binding energy of APTES and GOPES on the SiO2 surfaces.
| Model | Total energy (kJ mol−1) | Binding energy (kJ mol−1) |
|---|---|---|
| GOPES | −64.3522 × 106 | −149.4671 × 103 |
| APTES | −64.9352 × 106 | −148.7973 × 103 |
The good linkage of APTES on the SiN surface is also confirmed by the computational calculation results. The computational calculation revealed that the interaction of the APTES molecules and the silica surface had favorable energy so that the silanization and immobilization with APTES were more effective than with GOPES.
4. Conclusions
In conclusion, the computational calculations showed that the absorption of the APTES molecules on the SiN surfaces was better than GOPES. The experimental characterization results of the modified SiN surfaces revealed that the immobilization of MACF antibody with APTES–GTA modification was more effective than with GOPES. The results also confirmed the good linkage between APTES molecules and the SiN surfaces was attributed to the surface modification efficiency and the enhanced antibody immobilization.
Acknowledgments
The authors would like to thank Ministry of Science and Technology of Vietnam (MOST) for financial support.