Abstract
In this study, the arbuscular mycorrhizal fungus (G. mosseae) and endosymbiont (P. indica) colonized Zea mays were treated with calcium phosphate nanoparticles (CaPNPs) and evaluated for their plant growth promotion efficiency. It was observed that CaPNPs in combination with both G. mosseae and P. indica are more potent plant growth promoter than independent combinations of CaPNPs + G. mosseae, CaPNPs + P. indica or CaPNPs alone. The fluorimetric studies of treated plants revealed that CaPNPs alone and in combination with P. indica can enhance vitality of Zea mays by improving chlorophyll a content and performance index of treated plants. Hence, we conclude that CaPNPs exhibit synergistic growth promotion, root proliferation and vitality improvement properties along with endosymbiotic and arbuscular mycorrhizal fungi, which after further field trials can be developed as a cost-effective nanofertilizer with pronounced efficiency.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Agriculture is the cornerstone of national economies of many developing countries in general and India in particular. The crop production and global food security are greatly dependent on fertilizer input constituting nitrogen, phosphorus and potassium, as it supplements soil nutrients, promotes plant growth and increases crop productivity [1]. Low solubility of natural phosphorus (P)-containing compounds in soil leads to heavy use of P-containing fertilizers such as superphosphate of lime in kg/ha. However, the excessive use of P fertilizers is likely to cause micronutrient imbalance, eutrophication, leaching and ground water contamination [2]. Therefore, in order to overcome these impediments and to pave the way for advancement in agriculture, suitable agronomical practices combined with advanced technology should be practiced [3]. In this direction, the solution to overcome drawbacks of traditional agronomy may be nanotechnology. It has marked a new interdisciplinary enterprise by converging science and engineering into agriculture and food systems. Nanotechnology in agriculture has resulted into a shift from quantity to quality and has helped in the remediation of polluted sites [4]. The revolution implemented by nanotechnology in agriculture is aptly termed agronanotechnology [5]. It is a derivative of nanobiotechnology, which includes nanomaterials such as nanoparticles used as nanofertilizers and nanopesticides, aiding in substantial water and nutrient resource utilization, improvement in crop production and management, reduction of losses due to pests and diseases and prevention wastage of fertilizers as well as protection from environmental damages. Recently, a wide range of nanoparticles have been attracting immense attention in agronanotechnology due to their plant growth promoting activity. For instance, Dhoke et al [6] have investigated effect of ZnO, FeO and ZnCuFe-oxide nanoparticles on the growth of mung (Vigna radiata) seedlings. Zaberzadeh et al [7] have shown improved water deficiency tolerance capacity by TiO2 nanoparticles in wheat plants with increased seed gluten and starch content. Hong et al and Yang et al [8, 9] have also reported nano-TiO2 mediated improvement in photosynthesis and nitrogen metabolism in spinach plant. By modulating antioxidant status of Brassica juncea, silver nanoparticles were reported as plant growth promoting nanomaterials [10]. Similarly, iron oxide nanoparticles [11], nano iron chelate fertilizers [12], and nano-carbon [13] were evaluated for their effect on plant growth parameters, stress tolerance inducing capacity, fruit yield capacity, and early germination and flowering, etc.
Arbuscular mycorrhizal fungi are said to be a crucial factor in mobilization of soil phosphorus to roots which promote host growth [14]. For effectiveness of mycorrhization on vitality (performance) of photosynthetic system II (PS II), Strasser et al [15] have coined the term, 'biophysical phenomics'. Arbuscular mycorrhizal fungi, Glomus mosseae (G. mosseae) in synergism with Fusarium equiseti have shown plant growth enhancement effect and prevention from anthracnose and damping-off diseases when inoculated to cucumber plant [16]. Similar type of synergistic activity of G. mosseae and plant growth promoting fungi Penicillum simplicissimum in prevention of damping off disease in cucumber plant was demonstrated [17]. Piriformospora indica (P. indica) is a newly discovered arbuscular mycorrhiza-like root colonizing fungus, which also possesses growth promoting potential [18–20]. Prasad et al [21] have demonstrated positive influence of P. indica on biomass enhancement and antioxidant activity when inoculated with Bacopa monnieri. Similarly, co-cultivation of P. indica with barley in cool climate was resulted with hastening of flowering and significant increase in grain yield [22]. Kedar et al [23] have also reported the increase in biomass and content of Chl a in plants inoculated with endophytic Phoma spp.
In this study we assessed the effect of calcium phosphate nanoparticles (CaPNPs) on Zea mays inoculated with P. indica and G. mosseae.
2. Materials and methods
2.1. Materials
Sodium citrate, calcium chloride, dibasic sodium phosphate and half Murashige and Skoog media were purchased from Hi Media, Mumbai, India. All the solutions and glasswares were autoclaved before following the protocol.
2.2. Mycobionts
The culture of P. indica (Pi) was received as a gift from Dr Ajit Varma, Amity Institute of Herbal and Microbial Studies, (Noida), India. G. mosseae (Gm) was procured from Department of Biotechnology, S. G. B. Amravati University (Maharashtra), India and maintained as soil culture. The fungus P. indica was maintained on Kaefer's medium [24] at 25 °C for 8 days at static condition in the incubator. P. indica grew in concentric circles and appeared yellow-brown on sterile Kaefer's plate, while in Kaefer's broth it appeared as small whitish balls.
2.3. Synthesis of calcium phosphate nanoparticles
Calcium phosphate nanoparticles (CaPNPs) were synthesized by co-precipitation method [25]. 15.6 mM sodium citrate was pumped in a sterile beaker in which 12.5 mM calcium chloride and 12.5 mM dibasic sodium phosphate were added simultaneously by rapidly pumping both the solutions using sterile syringes. Mixture was stirred at 2000 rpm for 48 h at 4 °C, and sonicated for 30 min at 20 Hz. The final nanoparticle solution was stored at 4 °C for further studies.
2.4. Characterization of calcium phosphate nanoparticles
2.4.1. Nanosight analysis
Nanoparticle tracking analysis of the sample was performed by Nanosight LM-20 (UK), coupled with Nanosight NTA 2.0 analytical software program to determine the average size of the synthesized nanoparticles. Sample preparation was done by dispersing 5 μl of colloidal nanoparticles to 2 ml of sterile injection water.
2.4.2. Zetasizer analysis
Measurement of the zeta potential of the synthesized nanoparticles was carried out with the aid of Malvern Zetasizer 90 (ZS 90, USA). Sample preparation was done by diluting 30 μl of colloidal sample with 2 ml of sterile injection water. The mixture was sonicated for 15 min at 20 Hz to break large particles followed by filtration of mixture using a 0.22 micron filter.
2.4.3. FTIR spectroscopy studies
Sample preparation: 30 μl of colloidal CaPNPs were added to a pinch of KBr powder and incubated in hot air oven till the powder dried up. The dried powder was then used for FTIR analysis (Perkin-Elmer FTIR-1600, USA) in the range of 400–4000 cm−1 at a resolution of 4 cm−1.
2.5. Optimization of CaPNPs for growth promotion effect on maize plants
Surface sterilized Zea mays (Z. mays) seeds were germinated in sterile half Murashige and Skoog solid medium (containing sterile CaPNPs in different concentrations, 8–20 μg mL−1 as replacement of growth hormones). The plants were incubated in plant tissue culture laboratory for one week after inoculation.
2.6. Assessment of effect of CaPNPs + innoculum on maize plants in pot
The seeds of Z. mays were surfaced sterilized using 70% alcohol and 4% sodium hypochlorite for 1 min each. 3–5 seeds were potted in surface sterilized pot containing autoclaved soil and sand (3:1) and kept at room temperature in natural light for 12 h. Three seedlings of Z. mays were retained in each pot. Two weeks old experimental seedlings were inoculated with 1 ml of P. indica broth, 10 ml CaPNPs of the concentration 16 μg mL−1 and 5 gm of G. mosseae soil culture in three holes near the roots. Hewitt's solution [26] was applied to both control and experimental plants at interval of 10 days. However, only experimental plants were treated with CaPNPs + mycobionts at interval of 10 days. The treatments were given as follows:
- Set 1: Control
- Set 2: Calcium phosphate nanoparticles (CaPNPs)
- Set 3: CaPNPs + G. mosseae (CaPNPs + Gm)
- Set 4: CaPNPs + P. indica (CaPNPs + Pi)
- Set 5: CaPNPs + G. mosseae + P. indica (CaPNPs + Gm + Pi)
2.7. Biomass analysis
The one week old seedlings grown under tissue culture condition were taken out and root length and shoot height were measured. For evaluation of effect of NPs + innoculum on biomass, roots and shoots grown in pots were harvested after 45 days and heights and fresh weights of roots and shoots were recorded. For dry weight, roots and shoots were dried in oven at 50 °C up to the attainment of constant weight and dry weights were measured.
2.8. Study of root colonization
To study the roots colonization, roots were stained by standard method described by Phillips and Hayman [27]. The fine feeder roots were washed thoroughly in running tap water and cut into 1 cm pieces. The latter were treated with 10% KOH solution and subjected to an overnight incubation. Thereafter, the root pieces were washed 3–5 times with sterile distilled water and treated with 1% HCl for 3–4 min to make the sample acidic. Finally, the roots were stained with 0.05% cotton blue. The infected root pieces were examined under a light microscope (Axio Scope, A1, Carl Zeiss, US) at 40 × magnification.
2.9. Measurement of chlorophyll a fluorescence
Chlorophyll a fluorescence measurements were performed by Handy-PEA (Hansatech Instrument Pvt. Ltd, UK), a fluorimeter with high resolutions of 12 bit s−1. Prior to measurements, the samples were incubated in dark for 15 min followed by exposure to continuous light (650 nm peak wavelength, 3000 μmol photons-2 s-1 maximum light intensity) emitted by an array of three light emitting diodes focused on circular area of 5 mm diameter on sample surface. The first observations were recorded after seven days of seed germination. Subsequently, three more observations were recorded at an interval of seven days. Vitality and efficiency of the plants along with the photosynthetic activities (performance index, specific fluxes and yields) were analyzed with the aid of software Biolyzer.
3. Results and discussion
In present study CaPNPs were synthesized by employing co-precipitation [25]. For this, the calcium chloride solution was added to dibasic sodium phosphate which leads to gradual precipitation of the later which was further reduced into nanoparticles. These chemosynthetic CaPNPs were tested on Z. mays colonized by P. indica and G. mosseae. Here, CaPNPs were expected to promote growth of test plants, while mycorrhiza like P. indica and G. mosseae were inoculated to improve nutrient uptake and growth of the test plant.
3.1. Characterization of calcium phosphate nanoparticles
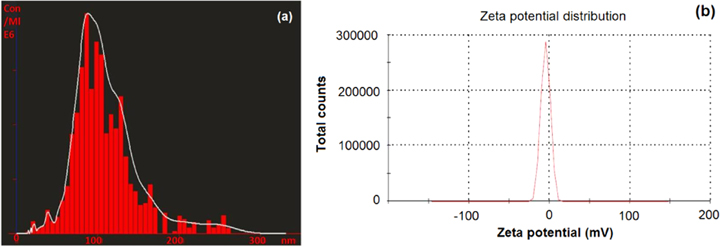
The nanoparticle tracking analysis determines size of nanoparticles by scattering of laser light beam on the surface of nanoparticles dispersed in colloidal solution and tracking Brownian motion of the same. Nanoparticle tracking analysis revealed CaPNPs with the average size to be 88 nm (figure 1(a)). The size reported by NTA 2.0 was well in accordance with the size of CaPNPs (70–80 nm) reported by Viswanathan et al [28].
Figure 1. (a) Nanosight LM-20 analysis of CaPNPs and (b) zetasizer analysis of CaPNPs.
Download figure:
Standard image High-resolution imageA sharp single peak in zetasizer analysis indicates quite monodispersed CaPNPs with −4.87 mV zeta potential (figure 1(b)). Further, negative zeta potential (−4.87 mV) revealed negative surface charge on CaPNPs denoting its incipient instability. Polydispersity index (0.282) revealed considerably heterogenous nanoparticle size distribution.
FTIR analysis of chemosynthesized CaPNPs was carried out to investigate different functionalities present on the surface of nanoparticles. The FTIR spectra of sodium citrate as a control show major bands at ∼1681, ∼1588, ∼1529, ∼1397 and ∼1280 cm−1 (figure 2 curve A). The peak at ∼1681 cm−1 can be assigned to C=O stretching of functional group alpha, beta unsaturated dialkyl ketones. Peak at ∼1588 cm−1 represented COO− asymmetric stretching of carboxylate group, while major band at ∼1397 cm−1 is attributed to COO− symmetric stretching of carboxylate group. Major band at ∼1529 cm−1 is attributed to CH stretching bands due to the ring stretching vibrations, whereas, OH in plane bending is evident from peak at ∼1280 cm−1.
Figure 2. FTIR spectra of (A) sodium citrate (control) and (B) capped CaPNPs.
Download figure:
Standard image High-resolution imageThe IR spectra of experimental calcium phosphate nanoparticles capped with sodium citrate exhibits peaks at ∼1754, ∼1622, ∼1529, ∼1470 and ∼1393 cm−1 (figure 2 curve B). The IR band at ∼1754 cm−1 can be assigned to the C=O stretching of alpha-keto ester, whereas, band at ∼1622 cm−1 can be attributed to C=O stretching of beta-keto ester [29]. The IR band at ∼1529 cm−1 represents the ring stretching of CH. The major IR bands at ∼1470 cm−1 and ∼1393 cm−1 can be assigned to CH3 bending and COO− symmetric stretching of carboxylate groups respectively. The IR spectra of CaPNPs expected to be capped by sodium citrate show C=O stretching, CH stretching, CH3 bending, COO− symmetric stretching. As most of the observed bonds of sodium citrate are conserved in the CaPNPs capped with sodium citrate (experiment) as well as the bonding stretching patterns suggest towards structural alterations occurred during capping mechanism thus strongly hinting towards effective capping of CaPNPs with sodium citrate.
3.2. Growth promotion activity of CaPNPs on Zea mays
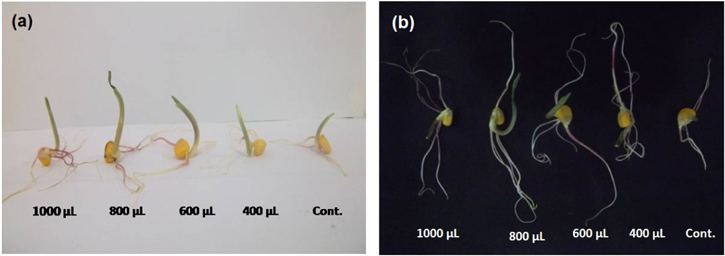
Zea mays was selected as it is commonly used for scientific study and is also a commercially significant cash crop. The optimum concentration of CaPNPs in order to enhance rooting was estimated in plant tissue culture system. Different concentrations of CaPNPs were screened in the range of 8–20 μg mL−1 (table 1). At certain optimum concentration, the seedlings displayed good growth over control, and beyond which, retardation of growth was observed in seedlings. Under controlled growth conditions, the effective growth at optimum concentration of 16 μg mL−1 was recorded (figures 3(a) and (b)). Moreover, inhibition of growth in seedlings treated with CaPNPs of the concentration 20 μg mL−1 was observed. This inhibition of growth can be attributed to accumulation of CaPNPs in roots.
Table 1. Average length of shoots and roots of Z. mays under tissue culture condition.
| Concentration of CaPNPs (μg mL−1) | Shoot length (cm) | Root length (cm) |
|---|---|---|
| Control | 2.25 ± 3.53 | 5.02 ± 3.06 |
| 8 | 3.25 ± 1.49 | 7.97 ± 1.59 |
| 12 | 3.87 ± 1.65 | 8.42 ± 3.01 |
| 16 | 5.75 ± 2.52 | 10.25 ± 0.64 |
| 20 | 3.25 ± 3.08 | 7.32 ± 1.94 |
All values are significant at 0.05% level of significance.
Figure 3. Effect of different concentrations of CaPNPs on length of (a) shoots and (b) roots of maize seedlings under tissue culture condition.
Download figure:
Standard image High-resolution imageIn pot experiments, endophytes in combination with CaPNPs were evaluated for their plant growth promotion on Z. mays seedlings. We observed that on treatment with nanoparticle-AMF combinations with maize seeds, a positive effect on shoot height, root length, fresh and dry weight of shoots and roots of treated plants was observed as compared to control plants. As evident from table 2, plants with tripartite combinations of CaPNPs + Gm + Pi attained maximum shoot height as compared to plants with independent combinations of NPs + AMF or nanoparticles alone. However, profuse rooting with maximum length was observed in plants treated with CaPNPs alone than plants inoculated with NPs + AMF and control. We conclude that CaPNPs play an important role for root differentiation as compared to inoculum. Dimpka et al [30] have also observed the profuse branching of roots of Triticum aestivum when treated with silver nanoparticles which was well in accordance with above study.
Table 2. Average length and weight of shoots and roots of Z. mays in pot experiment.
| Treatments | Shoot length (cm) | Shoot fresh weight (g) | Shoot dry weight (g) | Root length (cm) | Root fresh weight (g) | Root dry weight (g) |
|---|---|---|---|---|---|---|
| Control | 54.21 ± 5.34 | 11.99 ± 7.42 | 2.05 ± 0.47 | 35.83 ± 3.0.5 | 4.47 ± 1.18 | 1.09 ± 0.35 |
| CaPNPs | 56.94 ± 0.58 | 15.50 ± 2.83 | 2.25 ± 0.32 | 45.00 ± 2.64 | 7.81 ± 2.37 | 1.59 ± 0.28 |
| CaPNPs + Gm | 56.02 ± 1.59 | 14.08 ± 0.53 | 2.15 ± 0.65 | 37.00 ± 0.50 | 5.74 ± 0.04 | 1.24 ± 0.14 |
| CaPNPs + Pi | 57.41 ± 2.11 | 15.75 ± 2.05 | 2.22 ± 0.53 | 38.33 ± 4.93 | 6.94 ± 1.79 | 1.47 ± 0.43 |
| CaPNPs + Gm + Pi | 58.71 ± 0.76 | 16.72 ± 1.14 | 2.35 ± 0.46 | 42.00 ± 1.00 | 6.90 ± 1.40 | 1.09 ± 0.35 |
All values are significant at 0.05% level of significance.
Fresh and dry biomass of shoots and roots estimated with respect to different treatments in combinations of NPs + AMF for Zea mays plants after 45 days (table 2). Maximum fresh and dry weight of shoots was recorded for plants inoculated with CaPNPs + Gm + Pi and CaPNPs respectively. However, plants inoculated with independent combinations of CaPNPs, G. mosseae and P. indica didn't show any significant influence on fresh and dry weight of Z. mays shoots. The observations for fresh and dry weights were in accordance with length of shoots and roots, where, maximum plants with maximum root and shoot length revealed maximum values of biomass.
3.3. Root colonization of Zea mays plants
The colonization of both P. indica and G. mosseae was observed in roots of Z. mays. Spores of G. mosseae (figure 4(A)) and chlamydospores of P. indica (figure 4(B)) in aggregates were observed in root tissue of plant inoculated with combination of CaPNPs + G. mosseae + P. indica. Pear shaped spores of P. indica in cluster were observed in CaPNPs + P. indica root tissue (figures 4(C) and (D)). Also spores of G. mosseae were observed in CaPNPs + G. mosseae (figures 4(E) and (F)).
Figure 4. Colonization of roots by P. indica (Pi) and G. mosseae (Gm): (A) spores of G. mosseae in CaPNPs + Gm + Pi, (B) spores of P. indica in CaPNPs + Gm + Pi, (C) and (D) spores of P. indica in CaPNPs+Pi, (E) and (F) spores of G. mosseae in CaPNPs + Gm.
Download figure:
Standard image High-resolution image3.4. Chlorophyll a fluorescence assessment of Zea mays plants by Handy PEA
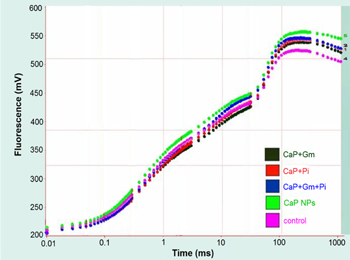
The analysis of phenomenological fluxes, performance index per absorbance (PIabs) and specific energy fluxes such as least dissipation per reaction center (DIo/RC), absorbance per reaction centre (ABS/RC), electron transport per reaction centre (ETo/RC), trapping per reaction center (TRo/RC) was performed by Chl a fluorescence. In the present study, the plants treated with CaPNPs showed maximum Chl a fluorescence followed by plants treated with CaPNPs + Gm + Pi, CaPNPs + Pi, CaPNPs + Gm and control (figure 5).
Figure 5. Fluorescence plot showing difference between Chl a fluorescence of treated and non treated plants of Zea mays.
Download figure:
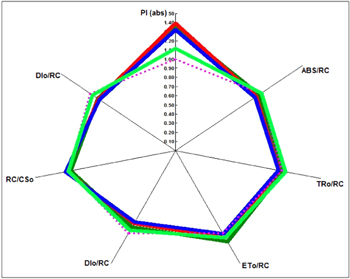
Standard image High-resolution imageThe radar graph showed the difference between control and experimental plants in order to their performance, dissipation, electron flux etc The plants inoculated with combination of CaPNPs + Pi showed highest (PIabs) and (DIo/RC) followed by CaPNPs + Gm, CaPNPs + Gm + Pi, CaPNPs and control (figure 6). The highest performance in treated plants was due to symbiotic association between plants, P. indica and calcium phosphate nanoparticles. Moreover, hypothetically, P. indica might have increased nutrient uptake capacity of plants. Increase in (ETo/RC), (TRo/RC), (ABS/RC) were observed for all treated plants as compared to control. Similarly, considerable decrease in DIo/CSo and DIo/RC was also recorded for all treated plants, which indicates that the CaPNPs, G. mosseae, and P. indica promote the plant growth.
Figure 6. Radar plot showing difference between performance index per absorbance and dissipation per reaction center of treated and control plants.
Download figure:
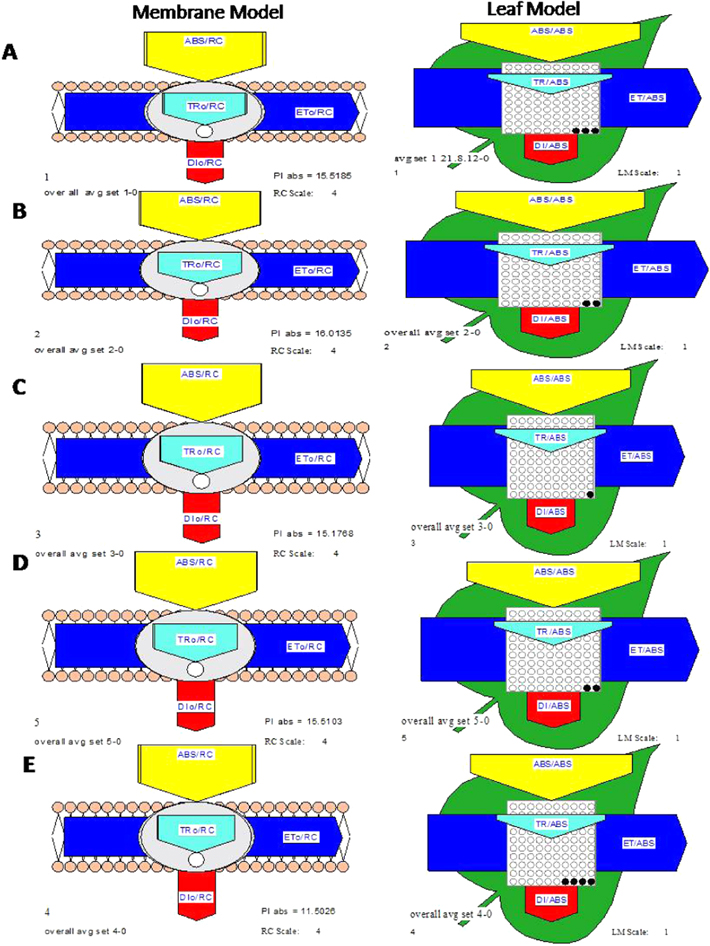
Standard image High-resolution imageThe pipeline model shows two different models, membrane and leaf model. The membrane model of CaPNPs + Pi followed by CaPNPs + Gm, CaPNPs, CaPNPs + Gm + Pi showed maximum performance index per absorption (PIabs) and (DIo/RC) as compared to control (figure 7). The black points in leaf model represent the fraction of inactive reaction centre. Moreover, among the treated plants, only plants treated with CaPNPs showed maximum fraction of inactive reaction centre followed by CaPNPs + Gm, CaPNPs + Pi, CaPNPs + Gm + Pi as compared to control. The specific fluxes (ABS/RC, TRo/RC and ETo/RC) of all treated plants were found to be increased and DIo/RC was decreased as compared to control. These all changes may be due to less deactivation of RC in treated plants than control which cause perturbation in electron transfer (ETo), so that the number of active RC controlled the intensity of the photosynthetic reactions. From the above observations, it can be concluded that the CaPNPs alone as well as in combination of P. indica influenced the photosynthesis promotion activity of Z. mays plants.
Figure 7. Pipeline model of Zea mays plants showing difference between performance index per absorbance (PIabs) and dissipation per reaction center (DIo/RC) and fraction of inactive reaction centre. (A) CaPNPs + Gm, (B) CaPNPs + Pi, (C) CaPNPs + Gm + Pi, (D) CaPNPs and (E) control.
Download figure:
Standard image High-resolution image4. Conclusion
The study indicates enhancement in the growth of the plants inoculated with CaPNPs + P. indica, CaPNPs + G. mosseae, CaPNPs + G. mosseae + P. indica and CaPNPs than control. These treatments enhanced the growth and vitality of Zea mays as interpreted from the fluorimetry studies by means of biosensing and probing photosynthesis as well as from root and shoot biomass analysis. In this designed experiment, the tripartite interactions among mycorrhiza, endophytic fungus and calcium phosphate nanoparticles revealed the synergism among all the three components. Calcium phosphate nanoparticles supplemented calcium and phosphate, the essential macronutrients required for profuse root proliferation, whereas the mycorrhiza-like fungus P. indica and the mycorhiza G. mosseae symbiotically promoted growth of experimental plants possibly by improved nutrient uptake of CaPNPs. Calcium phosphate nanoparticles alone have shown to promote growth and root maturation in Maize plants. Calcium phosphate nanoparticles may help in formulation of new nano growth promoter and nanofertilizers for agricultural use. Therefore, it could potentially help in reduction of the quantity of fertilizer applied to crops and contributing to precision farming as it reduces fertilizer wastage and in turn environmental pollution due to agricultural malpractices.
Acknowledgments
The authors gratefully acknowledge Professor (Dr.) Ajit Varma, Vice Chairman, Amity Science, Technology & Innovation Foundation (ASTIF) Amity University Uttar Pradesh, Noida, India for supply of culture of Piriformospora indica.