Abstract
Oxide dispersion strengthened (ODS) steels, manufactured by a mechanical alloying method, during the past few years, appear to be promising candidates for structural applications in nuclear power plants. The purpose of this work is to elaborate the manufacturing processes of ODS 13Cr steel with the addition of 1.0 wt% yttrium oxide through the powder metallurgy route using the high energy ball mill. Microstructural analysis by scanning electron microscopy (SEM), x-ray diffraction (XRD) and hardness testing have been used to optimize the technological parameters of milling, hot isostatic pressing and heat-treatment processes. The steel hardness increases with decreasing particle size of 13Cr ODS steel. The best hardness was obtained from more than 70 h of milling in the two tanks planetary ball mill or 30 h of milling in the one tank planetary ball mill and hot isostatic pressing at 1150 °C . The particle size of the steel is less than 100 nm, and the density and hardness are about 7.3 g cm−3 and 490 HB, respectively.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Oxide dispersion strengthening is a fruitful approach to improving the strength of ferritic/martensitic steels at high temperature as well as their resistances to corrosion and irradiation in view of their use in nuclear reactors. Oxide dispersion strengthened (ODS) steels with 0.3–1 wt% yttrium present better mechanical behavior than the base steels up to 500 °C and still maintain good properties up to 700 °C [1–3]. Production of ODS steels mainly comprises three processes: mechanical alloying (MA) by ball milling (MA technique), hot press-forming or extrusion, and recrystallizing heat treatment. The mechanical alloying involves the creation of an alloy by the intense mechanical deformation of a mixture of oxide and base steel powders. The powders are then consolidated by various metallurgical processes such as hot-isostatic pressing or extrusion. After mechanical alloying and consolidation, the steels have a very fine-grained microstructure and a very high hardness. Recrystallization heat-treatment is used to soften the alloy and to form a coarse grain structure, suitable for applications.
Mechanical alloying makes the combination of dispersion, solid solution and precipitation strengthening possible by mixing all of the constituents in powder form. During milling, the particles become trapped between the colliding balls, producing intense plastic deformation and fracture. The ductile steel powders are flattened and, where they overlap, the atomically clean surfaces just created weld together, building up layers of steel powders and dispersoids. These processes are repeated so that the mixed material becomes continually refined and homogenized until a true alloy powder is formed, leaving only the oxides dispersed in the solid solution. The performance of the ODS steels depends on the nano-scale oxide particle dispersion states, including the size, number density, microstructure and chemical composition [4, 5]. That is why the main purpose of the present work is to establish the optimal technological parameters of powder processing to enhance the performance of 13Cr ODS steel.
2. Experimental
2.1. Manufacture of ODS steel bars
The materials used in this study were commercial-grade 13Cr steel powder with a particle size of less than 45 μm and Y 2 O 3 powder (99% purity) with a particle of size less than 5 μm. The chemical composition of the 13Cr steel powder is given in table 1. First, steel powder and Y 2 O 3 powder were separately milled to the desired particle size (<1 μm for the steel powder and 50 nm for the Y 2 O 3 powder). Then, ODS steel powder was generated by high energy ball milling of milled 13Cr steel powder with 1 wt% nano-sized Y 2 O 3 powder. In this study, we used two types of planetary ball mills to compare their milling efficiency. It was recommended that, for oxidation protection purposes, the milling process was done under an Ar pressurized atmosphere or in an acetonic solution. The consolidation process was performed on a hot-isostatic press (HIP) at 1150 °C for 2 h under pressure of 170 MPa. This HIP condition was kept unchanged for all studied specimens. After pressing, the recrystallization heat treatment at 1150 °C for 2 h of the specimen bars was carried out in a vacuum heating furnace. Some of the technological equipment used in this study is shown in figure 1.
Figure 1 Planetary ball mill QM-2SP12-CL (a) and hot-isostatic pressing machine AIP6-30H (b).
Table 1. Chemical composition of commercial 13Cr steels powder.
| Cr | C | Si | Ni | Mn | Mo | P | S | O | Fe |
| 13.5 | 0.05 | 0.89 | – | 0.23 | – | 0.01 | 0.01 | 0.2 | Balance |
2.2. Materials characterization
The chemical composition and microstructure of the materials and specimens were characterized by energy dispersive spectroscopy (EDS) using SEM and XRD. SEM and EDS were used to characterize the particle size and dispersion of nano-sized Y 2 O 3 in the metallic matrix too. The density measurement was needed for the characterization of specimens after HIP and after the heat-treating process. Through density and mechanical testing the technological parameters for the fabrication of ODS steels were assessed to be optimal.
3. Results
3.1. Microstructural characteristics
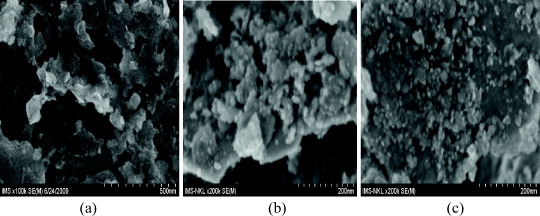
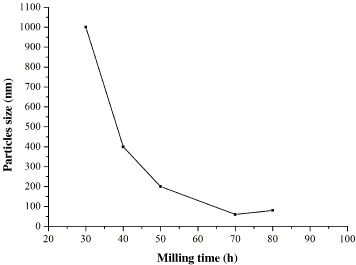
Figure 2 shows SEM images of the milling powders after different milling times and figure 3 illustrates the change in particle sizes during milling. Both show that during milling the particle size of the powders was continuously refined and nano-size grains occurred after 50 h of milling. When we used a planetary ball mill QM-2SP12-CL with a speed of 350 rpm, a grain size of less than 100 nm was obtained after 70 h of milling. When the milling time was longer than 70 h, the size seemed to be larger. This was caused either by agglomerating or flattening of the particles [4]. When we used the other ball mill with a speed of 500 rpm, a grain size of about 50 nm was obtained up to 50 h of milling. In [4] the minimum grain size of 47 nm was obtained after 12 h of milling with a milling speed of 800 rpm. This proves that with increasing milling speed, the minimum grain size is obtained earlier and the size is smaller.
Figure 2 SEM images of powders (a) after 30 h, (b) 50 h and (c) 70 h milling.
Figure 3 Changes in particle size as a function of milling time.
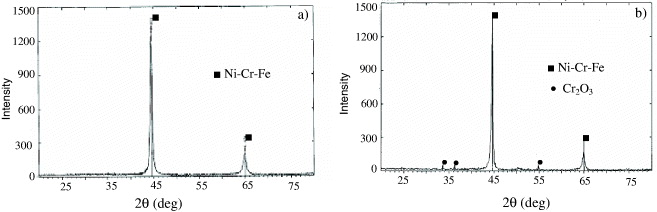
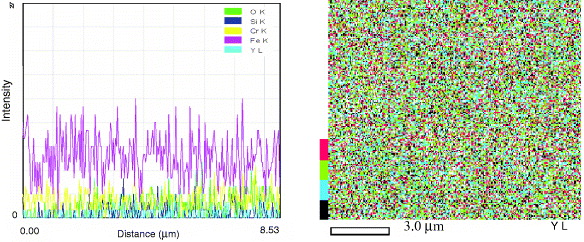
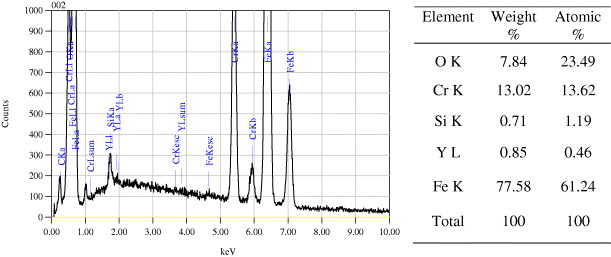
Figure 5 shows the distribution of Y 2 O 3 particles in the metallic matrix resulting from continuous milling and mixing of the mixture during milling. It shows the fine dispersion of Y 2 O 3 particles, homogeneously distributed in the studied cross-section of the specimen. An XRD pattern and EDS analysis presented in figures 4 and 6 also show that the total chemical composition of the studied ODS steel is kept unchanged during and after milling, press-forming and heat treating.
Figure 4 XRD patterns of the specimen before (a) and after (b) heat treatment.
Figure 5 SEM–EDS results for the yttrium element of a heat-treated specimen.
Figure 6 EDS results for the composition of a heat-treated specimen.
3.2. Mechanical properties
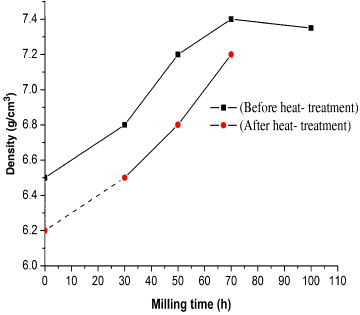
Figure 7 shows the density of the specimens as a function of milling time. It was found that the density of a specimen increases with increasing milling time. The high density of the ODS steel bar was obtained by good pressing, but it also strongly depended on the fine size of the pressing powder. In the case of unchanged pressing technological parameters, the specimen with the minimum particle size has the maximum density. Figure 7 also shows the differences in the densities of specimens before and after heat treatment. The decreasing density of the heat-treated specimen was caused by recrystallization during heat treatment.
Figure 7 The changes in density of specimens as a function of the milling time before and after heat treatment.
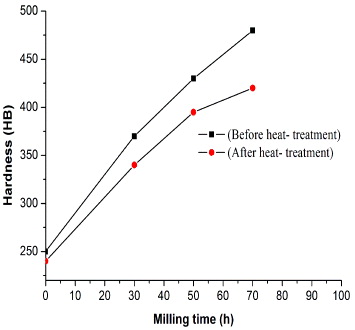
The hardness of the bars before and after heat treatment is shown in figure 8. The high hardness of the ODS 13Cr steel resulted from its fine grain size and from the dispersion of nano-size oxide particles in its matrix . This means that the finer grain size ODS steel was harder than that with a coarse grain size. Recrystallization made the ODS steel less hard, with suitable ductility for applications.
Figure 8 The changes in hardness of specimens as a function of the milling time before and after heat treatment.
4. Conclusions
One type of ODS 13Cr steel was fabricated through the powder metallurgy route, and some technological parameters of the fabrication process were established. The optimal milling time depends on the speed of milling. Higher speed need shorter milling times. In the case of using a planetary ball mill with a speed of 350 rpm, the minimum milling time is 70 h to obtain a steel particle size of less than 100 nm and Y 2 O 3 oxide finely dispersed in the steel matrix. ODS 13Cr steel consolidated from finer powder has a higher density and hardness. The density and hardness of ODS 13Cr steel fabricated by HIP from powder with a particle size of 100 nm are about 7.3 g cm −3 and 490 HB, respectively.
Acknowledgments
The support of the Vietnam Academy of Science and Technology is highly appreciated. The experiments and analysis in this study were carried out in the Laboratory of Advanced Metallic Materials COMFA and the National Key Laboratory of Electronic Materials of the Institute of Materials Science, Vietnam Academy of Science and Technology.
Footnotes
Report submitted to the 5th International Workshop on Advanced Materials Science and Nanotechnology IWAMSN, Hanoi, 9–12 November 2010.