Abstract
Non-stoichiometric nanocrystalline Li4Mn5O12 was synthesized using the sol–gel method with citric acid at a low-temperature. The effects of the pH condition on the structure, morphology and electrochemical properties were investigated. X-ray diffraction patterns and scanning electron microscopy images confirmed that all the samples crystallized in spinel structures with a particle size in the nanometric scale. Transmission electron microscopy images showed that particle size varied from 50–100 nm. The Li4Mn5O12 was tested as an electrode material in half-cell Li/Li4Mn5O12 and in full-cell vulcanized carbon/Li4Mn5O12 at a high rate charge–discharge. Among the samples, Li4Mn5O12 (prepared at pH = 9) exhibited the best electrochemical capacitive performance in an aqueous hybrid capacitor full-cell AC/Li4Mn5O12 as well as in an non-aqueous Li/Li4Mn5O12 half-cell. The specific sample delivered a power density of 20 F g−1 at a high discharge rate of 4 A g−1 and an energy density of 31.1 Wh g−1.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The hybrid capacitor or asymmetric capacitor, a hybridization of an electrochemical capacitor and rechargeable batteries, is based on a battery electrode (charged by a Faradic reaction) and an electrochemical capacitor electrode (with charge stored in the electrochemical double layer) [1]. Sometimes, the term 'lithium–ion' capacitor is used to describe a device using a graphite negative electrode and activated carbon (AC) based positive electrode in combination with a Li+ containing electrolyte [2]. For such devices, many different types of materials have been proposed for the Faradic component and lithium insertion compounds have been of interest due to their high specific charge and good cycling stability. For example, it has been reported that Li4Ti5O12 [3, 4], LiCoO2 [5, 6], LiMn2O4 [7, 8] and LiNi1/3Co1/3Mn1/3O2 [9] show a higher energy density in hybrid capacitors than the conventional electrochemical double layer capacitor.
Lithium manganese oxides have been investigated the most for use as cathode materials for lithium batteries (LIBs) and supercapacitors because of their facile preparation, abundance, low cost and non-toxicity [7, 8, 10–13]. Among these oxides, the Li4Mn5O12 phase was applied in both LIBs and hybrid capacitors [4, 14, 15]. Recently, Jiang et al [16] used a spray-drying-assisted solid-state reaction to synthesize nano-crystallites of Li4Mn5O12, which presents a specific capacity of 120 mAh g−1 at the rate C/2. Hao et al [17] have reported the preparation of Li4Mn5O12 by a sol–gel process and calcination at 600 °C. The aqueous asymmetric supercapacitor AC/Li4Mn5O12 presents a specific capacitance (Csp) of 43 F g−1 in the potential range 0–1.4 V at the rate of 100 mA g−1. Kim et al [18] have synthesized nano-Li4Mn5O12 using the solution combustion method at 400 °C for 5 h. They have developed a non-aqueous asymmetric supercapacitor AC/Li4Mn5O12 for which Csp reached 55 F g−1 in the potential range of 1.0–2.5 V.
Theoretically, Li4Mn5O12 has a specific capacity of 163 mAh g−1 in the 3 V region and no capacity in the 4 V region due to the valence 4+ of the manganese ion. Non-stoichiometric Li4Mn5O12 exhibited oxidation–reduction in the 4 V region due to the existence of Mn3+, and typically a low discharge capacity of 60 mAh g−1 at the C/20 rate in 3.5–4.5 V [18]. Therefore, we propose using non-stoichiometric and nanocrystalline Li4Mn5O12 to increase the power density of hybrid capacitors as well as their rate charge–discharge capability. In this work Li4Mn5O12 was synthesized using the sol–gel method at low-temperature. We investigated the effects of pH conditions on the structure, morphology and electrochemical properties of the Li4Mn5O12 synthesized materials. Cell performance and cycling ability were investigated in both non-aqueous and aqueous electrolytes.
2. Experimental details
2.1. Sol–gel synthesis
Nanocrystalline Li4Mn5O12 was synthesized using the sol–gel method with the precursors Li(CH3COO).2H2O (Sigma Aldrich, 99.9%) and Mn(CH3COO)2 (Sigma Aldrich, 99.9%). The first step in the sol–gel process was to dissolve and agitate the precursors (with a molar ratio of Li:Mn = 4:5) into a mixture of ethylene glycol, ethanol and distilled water (with a volume ratio 6:3:1). Then, the citric acid solution (as a chelated agent) was added to the mixed solution under stirring, the ratio of citric acid to total metal ions was used in 1:1. The pH of solution was then adjusted using NH3 solution (pH = 8–11). The 'sol' solution was heated and stirred at 100 °C until gel formation was observed. Finally, the gel was heated at 300 °C for 5 h in air.
The structures of the materials were identified using x-ray diffraction (XRD) using a D8-Advance (Bruker) diffractometer with a copper anode (λKα = 1.5408 Å), XRD patterns were collected in the range 10–70° (0.029° s−1). Lattice parameters were calculated using the software Celref. The morphology and the grain size distribution were determined using field emission scanning electron microscopy (FE-SEM; S4800 Hitachi, Japan) and transmission electron microscopy (TEM; JEOL JEM 1400).
2.2. Electrochemical measurements
The electrode paste was prepared by mixing Li4Mn5O12 or active carbon (AC) powder with acetylene black, graphite and polytetraflouroethylene at a weight ratio of 80:15:5. The paste was laminated to 0.1 mm thickness, cut into pellets with a diameter of 10 mm and dried at 130 °C under a vacuum.
The electrochemical properties of nanocrystalline Li4Mn5O12 were evaluated using cyclic voltammetry (CV) and the charge–discharge test at a high rate. The CV was performed in the 1 M Li2SO4 aqueous solution using a three-electrode cell where the working electrode is platinum coated with Li4Mn5O12, the counter electrode is platinum and the reference electrode is Ag/AgCl (KCl saturated), respectively. The Li/Li4Mn5O12 half-cell test was accomplished using Swagelok cells. The electrode Li4Mn5O12 and Li foil were used as the positive and negative materials in the half-cell and a solution of 1 M LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) at a ratio of EC:DCM = 2:1 was used as the electrolyte.
The asymmetric hybrid capacitors AC/Li4Mn5O12 in the Swagelok cell consisted of Li4Mn5O12 as the cathode and AC as the anode. The high rate charge–discharge test was performed in the potential window 0–1.4 V for the aqueous solution 1 M Li2SO4 and 0–3 V for the non-aqueous solution 1 M LiPF6/EC:DMC (2:1). All electrochemical experiments were carried out using a MPG2 apparatus (Biologic, France).
3. Results and discussion
3.1. Structure and morphology of nanomaterial
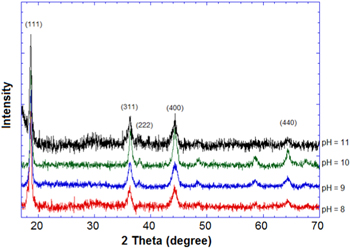
The Li4Mn5O12 phase is normally crystallized in the spinel phase with a cubic face center structure (space group: Fd3m): lithium ions are located simultaneously at the tetrahedral 8a and the octahedral 16d sites, manganese ions are distributed at the octahedral 16d sites and the oxygen ions occupy the 32e sites. As shown in figure 1, the XRD patterns of all the samples could be identified to a pure phase of spinel structure Li4Mn5O12 (JCPDS: No. 46-0810) without the emergent second phase. According to the XRD results, the lattice parameters of the samples were calculated to be 8.1904 Å, 8.1990 Å, 8.1795 Å and 8.1893 Å, respectively (table 1). According to previous reports, the literature value of the lattice parameter is 8.161 Å [14, 17], thus the synthesized samples were non-stoichiometric. The oxidation value of manganese and the ratio Li:Mn were determined by oxidation–reduction titration and atomic absorption spectroscopy. As can be seen in table 1, all samples exhibiting manganese oxidation have values less than 4, the chemical formula was thus identified. It was apparent that the preparation of stoichiometric Li4Mn5O12 was still not realized, in particular at temperatures lower than 600 °C [15]. The non-stoichiometric compounds are able to intercalate lithium reversible not only in the 3 V range but also in 4 V range at the expense of the reduction of Mn4+ and Mn3+.
Figure 1. XRD patterns of samples of Li4Mn5O12 prepared at different pH values.
Download figure:
Standard image High-resolution imageTable 1. The lattice parameters of the Li4Mn5O12 samples.
| Sample | pH | a (Å) | ν (Mn) | Chemical formula |
|---|---|---|---|---|
| S08 | 8 | 8.1904 | 3.54 | Li3.51Mn2IIIMn3IVO10.8 |
| S09 | 9 | 8.1990 | 3.57 | Li3.48MnIII1.8MnIV3.2O10.8 |
| S10 | 10 | 8.1795 | 3.73 | Li4.11MnIII0.75MnIV4.25O11.7 |
| S11 | 11 | 8.1893 | 3.62 | Li3.4MnIII1.25MnIV3.75O11.1 |
The XRD patterns of the low-temperature Li4Mn5O12 were quite large (figure 1); it was considered that the samples crystallized in small particle sizes. The small particles and high surface area played important roles in improving the electrochemical double layer capacitance (the non-Faradic reaction).
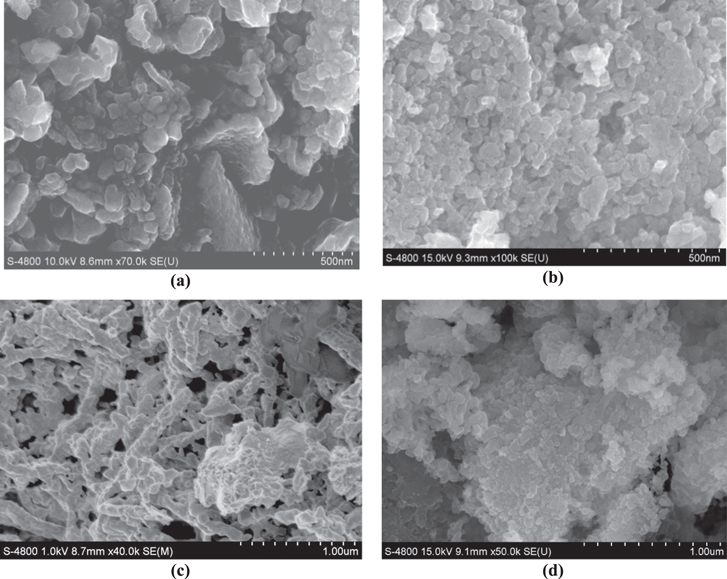
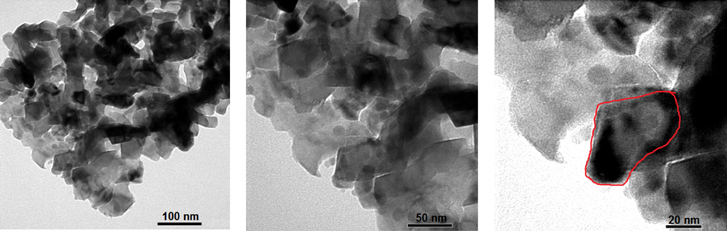
SEM images of the Li4Mn5O12 samples are shown in figure 2. The morphology of the Li4Mn5O12 particles was affected by the pH value and the particles appear to be more regular and smaller with low pH values (pH = 8, 9). As can be seen in the figure 3(b), the sample S09 had a homogenous grain distribution, and the particle size fell into the nanometric dimension scale. The agglomeration of particles was clearly observed for other samples at pH values = 8, 10, 11. The TEM images of sample S09 confirmed that this sample had a polycrystalline structure and the particles size varied from 50–100 nm (figure 3).
Figure 2. SEM images of Li4Mn5O12 prepared at different pH values: (a) pH = 8, (b) pH = 9, (c) pH = 10 and (d) pH = 11.
Download figure:
Standard image High-resolution imageFigure 3. TEM images of Li4Mn5O12 at pH = 9 (sample S09).
Download figure:
Standard image High-resolution image3.2. Electrochemical characterization
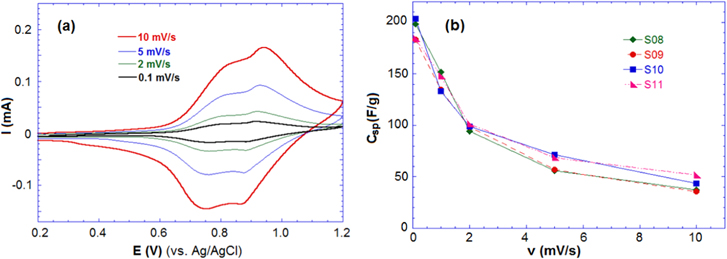
The CV was performed by a three-electrode cell in the range 0.2–1.2 V (versus Ag/AgCl) for the aqueous 1 M Li2SO4. Figure 4(a) shows the CV curve of sample S09 at various scan rates (v) of 0.1, 1.0, 2.0, 5.0 and 10.0 mV s−1. Two-redox reversible peaks were observed in all the CV curves, which confirms the reversible intercalation of Li+ ions into the host Li4Mn5O12, corresponding to the redox reaction Mn(III) ⇄ Mn(IV). At low scan rates, two broad redox peaks could be observed: two anode peaks occurring at 0.91 and 1.04 V and two cathode peaks appearing at 0.83 and 0.93 V. Following the increase of the scan rate, it was observed that the peak positions has shifted around 300 mV and the redox peaks gradually became inconspicuous.
Figure 4. (a) CV curves of sample S09 and (b) the specific capacitance Csp of samples at different scan rates in 1 M Li2SO4.
Download figure:
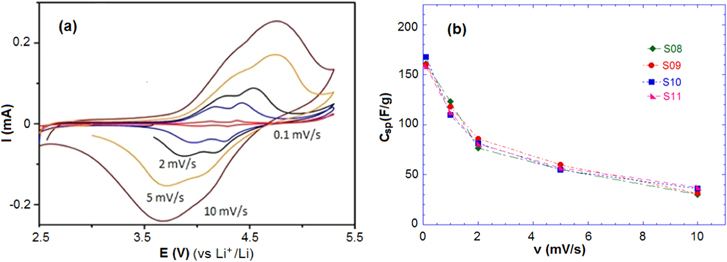
Standard image High-resolution imageThe CV characterization was also performed in non-aqueous 1 M LiPF6/EC:DMC (2:1) solution in the range 2.5–5.0 V. Figure 5(a) shows two oxidation–reduction peaks, similarly to the CV in aqueous solution. These peaks are also related to the redox reaction Mn(III) ⇄ Mn(IV). Increasing the scan rate results in shifted potentials of the oxidation–reduction peaks as well as the increase of the peak current.
Figure 5. (a) CV curve of sample S09 and (b) the specific capacitance Csp of samples at different scan rates in 1 M LiPF6/EC:DMC.
Download figure:
Standard image High-resolution imageThe specific capacitance (Csp) is calculated by using half the integrated area of the CV curve to obtain the charge (Q), and subsequently dividing the charge by the mass of the electrode (m) and the potential window width (ΔV)
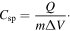
The Csp of samples at different scan rates in aqueous and non-aqueous electrolyte are presented in table 2. The values for Csp in the aqueous solution are higher than the values in the non-aqueous solution due to the high conductivity of the 1 M Li2SO4 solution. In the aqueous solution, the samples reached high specific capacitances of 200, 190, 185 and 203 F g−1 at the lowest scan rate (0.1 mV s−1), and Csp decreased with increasing of scan rate. At a scan rate of 10 mV s−1, the Csp of the samples was found to be around 50 F g−1 for both the aqueous and non-aqueous solution. The CV results indicate that the charge storage of Li4Mn5O12 relies on the mechanism of insertion/extraction of Li+ ions and the intercalation of Li+ occurring simultaneously on the surface and in the internal structure of the material.
Table 2. Specific capacitances of samples in aqueous and non-aqueous solutions.
| Specific capacitance Csp (F g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| in 1 M Li2SO4 | in 1 M LiPF6/EC:DMC | |||||||
| Scan rate v (mV s−1) | S08 | S09 | S10 | S11 | S08 | S09 | S10 | S11 |
| 10 | 37.2 | 35.9 | 43.7 | 51.9 | 30.18 | 31.48 | 36.06 | 37.56 |
| 5 | 55.9 | 57.1 | 71.7 | 68.7 | 55.46 | 59.91 | 55.13 | 57.04 |
| 2 | 94.4 | 98.2 | 98.7 | 101.7 | 76.76 | 85.93 | 81.45 | 80.66 |
| 1 | 151.6 | 134.7 | 132.9 | 147.9 | 123.33 | 117.82 | 109.60 | 112.24 |
| 0,1 | 198.3 | 183.2 | 203.4 | 184.4 | 161.23 | 160.18 | 167.74 | 158.65 |
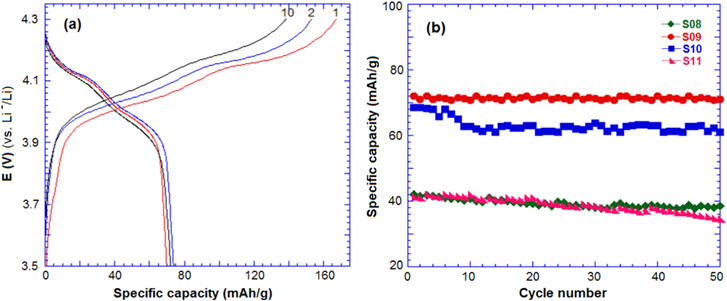
Figure 6(a) shows the charge–discharge profile of samples at a C/5 rate in a half-cell. The charge–discharge curves showed two potential plateaus at 4.1–4.2 V and 4.1–3.9 V, corresponding to two oxidation–reduction peaks in the CV curve. All the samples showed stable performance after 50 cycles and sample S09 exhibited the best discharge capacity of 72 mAh g−1. Kim et al [18] reported that the Li4Mn5O12 phase, prepared at 400 °C for 5 h, exhibited the discharge capacity of 46 mAh g−1 at a C/5 rate.
Figure 6. (a) Charge–discharge curve of sample S09 and (b) the cycle performance of the samples in a half-cell with lithium at a C/5 rate.
Download figure:
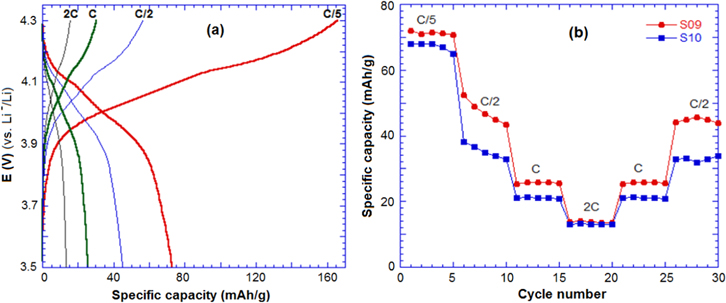
Standard image High-resolution imageThe charge–discharge performances of S09 and S10 were evaluated under different rates: C/5, C/2, 1C and 2C (figure 7). At the low rates (C/5, C/2), S09 and S10 reached a specific capacity around 70 mAh g−1, while they presented a value of 12 mAh.g−1 at the high rate 2C. The sample S10 exhibited a lower discharge capacity than S09 due to the high content of Mn4+ in the chemical formula.
Figure 7. (a) Charge–discharge curve of sample S09 and (b)the cycle performance of S09 and S10 in a half-cell with lithium at high rate charge–discharge.
Download figure:
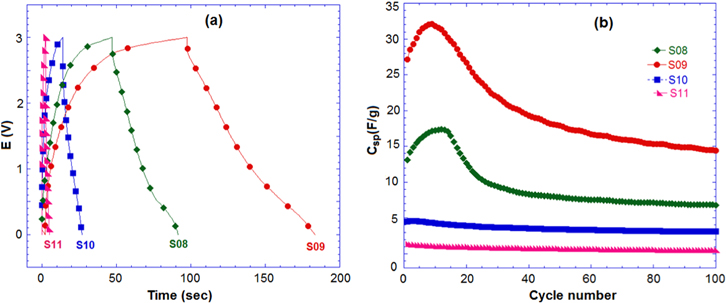
Standard image High-resolution imageTo investigate the performance of samples in a full-cell, the charge–discharged test of the hybrid capacitor AC/Li4Mn5O12 was performed at rate of 4 A g−1 in the aqueous and non-aqueous solutions. In the aqueous electrolyte (figure 8), the AC/Li4Mn5O12 hybrid capacitor shows a relative high specific discharge capacitance and excellent cycling stability of capacitance during 200 cycles. The Csp obtained for S08, S09, S10 and S11 were 14 F g−1, 21 F g−1, 6 F g−1 and 12 F g−1, respectively. The aqueous full-cell AC/Li4Mn5O12 (S09) hybrid capacitor exhibited a specific energy of 31.1 Wh kg−1. The highest Csp of S09 could be attributed to the homogenous size distribution and nanometric particles compared to other samples presenting the agglomeration phenomena.
Figure 8. (a) Discharge curve and (b) cycle performance of hybrid capacitors AC/Li4Mn5O12 at 4 A.g−1 in 1 M Li2SO4.
Download figure:
Standard image High-resolution imageFigure 9 shows the charge–discharge curve and cycle performance of a non-aqueous hybrid capacitor with the samples. S09 shows a good Csp of 33 F g−1, corresponding to a specific energy of 100 Wh kg−1. However, the Csp decreased below 15 F g−1 after 100 cycles.
Figure 9. (a) Discharge curve and (b) cycle performance of hybrid capacitors AC/Li4Mn5O12 at 4 A g−1 in 1 M LiPF6/EC:DMC.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, Li4Mn5O12 nano-crystallites were successfully synthesized at a low temperature using the sol–gel method with citric acid as the chelated agent. Sample S09 prepared at pH = 9 has a regular grain distribution and the grain's size varies from 50–100 nm. S09 can intercalate Li+ ions in aqueous and non-aqueous electrolytes. This material exhibits an excellent specific capacitance (200 F g−1) in 1 M Li2SO4 and a high specific capacity (76 mAh g−1) in 1 M LiPF6/EC:DMC. At a high rate charge–discharge (4 A g−1) the aqueous full-cell hybrid capacitor AC/Li4Mn5O12 has shown good performance and stability with a specific capacitance of 20 F g−1 during 200 cycles in the potential range 0–1.4 V.
Acknowledgments
The authors would like to thank VNU-HCM for grant HS2013-76-01. We thank Dr Nguyen Tri Tuan, Can Tho University for his support in the XRD analysis. We also acknowledge Academician Nguyen Van Hieu for his discussion and support.