Abstract
Nanostructured polypyrrole film was synthesized onto a copper electrode in solutions of oxalic and salicylic acids and their buffers. The electrooxidation of pyrrole to form polypyrrole film and the electroreduction of nitrate and nitrite ions at synthesized Ppy modified copper electrodes (Ppy/Cu) in potassium chloride aqueous solutions were studied using chronoamperometry. The nanoporous structure of the synthesized Ppy films was characterized by scanning electron microscopy (SEM). Nitrate and nitrite reduction were performed by an electrochemical method under potentiostatic conditions. The Ppy/Cu electrodes prepared in the oxalate buffer and salicylic acid solutions perform more stable catalytic activity for nitrate reduction; their service life is about ten times longer than that for the electrodes prepared in oxalic acid solution. After 20 h of electrolysis, the nitrite was reduced completely with 100% efficiency and the nitrate was reduced with 35% efficiency.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nitrates are a potential human health threat, especially to infants, causing the condition known as methemoglobinemia, also known as 'blue baby syndrome'. Survey results show that approximately 8–10% of blue baby syndrome cases result in death of the infant. Exposure to high levels of nitrate in drinking water has been linked to a variety of effects, ranging from hypertrophy (enlargement of the thyroid) to 15 types of cancer, two kinds of birth defect and even hypertension. Since 1976, there have been a number of different epidemiology studies conducted in 11 different countries that show a definite relationship between increasing rates of stomach cancer and increasing nitrate intake. The facts speak for themselves: increasing levels of nitrates in our groundwater are slowly poisoning our society.
To protect humans from the life-threatening effect of nitrates, numerous researches have been reported to remove nitrate from drinking water and groundwater, the primary water supply for agriculture, industrial and animals. There are several treatment methods for removing nitrate, such as ion exchange, reverse osmosis and electro-dialysis, which can be separated into two main groups: methods using microorganisms and electrochemical methods. The electrochemical approach is receiving more and more attention due to its relatively simple process (no requirement of chemicals, no sludge to be disposed of, small area, and typically operating at low temperature and atmospheric pressure), environment-friendly property and low cost [1–3].
Copper exhibits an advanced electrocatalytic activity in nitrate reduction compared to other materials, such as nickel, graphite and platinum [4]. Unfortunately, nitrate electroreduction of pure copper causes the formation of nitrite and ammonia, which are also toxic [4, 5]. Consequently, many studies in recent years have focused on various electrode materials in acidic [6, 7], alkaline [8–10] and neutral [11–14] media. However, the main problem of those researches on the process of the electrochemical reduction of nitrate is the production of more toxic side-products than nitrates, such as NO 2 − and NH 3. The application of a conducting polymer as a support for electrocatalytic microparticles has been the subject of numerous investigations. As one of the inherent conductive polymers, polypyrrole (Ppy) has been extensively studied as a modification layer for various sensors [15–19]. In our previous papers [20–24], Ppy exhibited catalytic activity for nitrate reduction and good selectiveness, i.e. avoiding nitrite formation. For those reasons, the Ppy/Cu electrode has been chosen for the nitrate electroreduction in this study.
In the previous studies [20–24], we synthesized the Ppy films onto the copper electrode in oxalic acid, oxalate buffer, salicylic acid and salicylate buffer solutions under certain conditions of anodic potential and electrolysis durations. After thermal treatment the synthesized Ppy/Cu electrodes were used for nitrate and nitrite reduction. Our purpose in this work was to study (i) the effect of the heat treatment on the morphology of the synthesized Ppy films on copper electrode, and (ii) the catalytic activity of Cu and Cu modified by Ppy in different electrolyte solutions towards nitrate and nitrite reduction.
2. Experimental
2.1. Preparation of the synthesized Ppy/Cu electrode
Copper disks (99.9%) molded in epoxy were used as the working electrode (exposure area is 0.01, 0.2 cm 2). The copper surface was polished with 1200 grit emery paper, washed in ethanol, cleaned with twice distilled water in an ultrasonic bath and dipped in 0.5 M HCl for about 30 min, then cleaned with twice distilled water again to eliminate HCl. This copper electrode was then used as the working electrode in the electro-deposition of polypyrrole on a copper electrode. The experiment was carried out at room temperature (27 °C±1 °C) under quiescent conditions by using a three-electrode, one-compartment cell with a potentiostat/galvanostat (PGS-HH9). A saturated silver chloride electrode (Ag/AgCl) was chosen as the reference electrode. Platinum wire was used as a counter electrode. The Ppy/Cu electrodes were prepared by electro-deposition (chronoamperometry) of Ppy from a solution of 0.1 M pyrrole (monomer) dissolved in different solutions, as listed in table 1, which are oxalate buffer (0.2 M oxalic acid and 0.05 M ammonium oxalate) and salicylate buffer (0.01 M salicylic acid and 0.005 M ammonium salicylate).
Table 1. Synthesized conditions of Ppy/Cu electrodes used for nitrate and nitrite electroreduction.
| Ppy/Cu-OA | Oxalic acid | 1.6 V | 60 |
| Ppy/Cu-OB | Ammonium oxalate buffer | 1.6 V | 60 |
| Ppy/Cu-SA | Salicylic acid | 2.0 V | 240 |
| Ppy/Cu-SB | Ammonium salicylate buffer | 2.0 V | 150 |
The prepared Ppy/Cu electrodes were first immersed in distilled water for 24 h and then dried in an oven at a temperature of 40–45 °C for 5 h. They were then immersed again in distilled water for three weeks before using for nitrate and nitrite electroreduction.
2.2. Electroreduction of nitrate and nitrite
Electroreduction of nitrate and nitrite was performed in a 200 ml one-compartment electrolytic cell using a potentiostat/galvanostat (PGS-HH9). A three-electrode was used with a Ppy/Cu working electrode with a 0.2 cm 2 exposed area, platinum wire counter electrode and saturated silver chloride (Ag/AgCl) reference electrode.
Cyclic voltammetry (CV) experiments were conducted using 0.15 M potassium chloride (KCl) solution as a supporting electrolyte. Sodium nitrate (NaNO 3) and sodium nitrite (NaNO 2) solutions were prepared just prior to use. The CV experiments were run with a potential scan rate of 0.01 V s −1. The electrochemical measurements were carried out in deaerated solutions in a pure nitrogen atmosphere.
The electrolysis processes (chronoamperometry) were conducted in the solution of 0.005 M sodium nitrate (NaNO 3) or 0.005 M sodium nitrite (NaNO 2) at −1.05 V for different periods of time. The nitrate and nitrite concentrations were analyzed with a UV-Vis spectrophotometer (Varian Cary 50).
The morphology of the synthesized material was investigated by scanning electron microscopy (SEM, JSM 6480LV, Jeol Co., Japan).
3. Results and discussions
3.1. The morphology of oxidized Cu surface and Ppy films synthesized in different electrolyte solutions
3.1.1. Oxidized Cu surfaces and Ppy films synthesized in oxalate buffer and salicylate buffer solutions
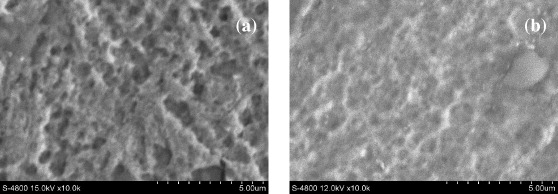
The morphologies of the Cu surfaces oxidized in oxalate buffer and salicylate buffer solutions are shown in figure 1. As can be seen, the Cu surfaces oxidized in two electrolyte solutions show different results (figures 1(a) and (b)). This might explain the difference in morphology of Ppy films formed in oxalate buffer (figure 2(a)) and salicylate buffer solutions (figure 2(b)).
Figure 1 SEM images of Cu surfaces oxidized in solutions of (a) oxalate buffer and (b) salicylate buffer.
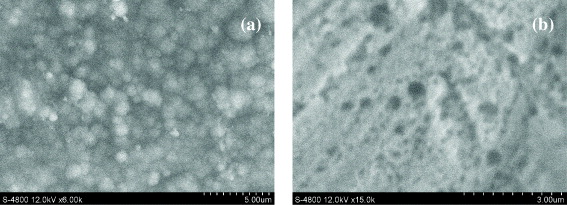
Figure 2 SEM images of Ppy films synthesized in solutions of (a) oxalate buffer and (b) salicylate buffer.
Figure 2 shows SEM images of Ppy films generated on Cu electrodes. Ppy appears to have instantaneous hemispherical deposits, which generate a film of nodular morphology (commonly called 'cauliflower-like' morphology) (figure 2(a)). The formed Ppy films were also confirmed by comparison with the SEM images of non-Ppy oxidized Cu surfaces in figure 1.
3.1.2. Effect of heat treatment of Ppy/Cu on the morphology of Ppy films
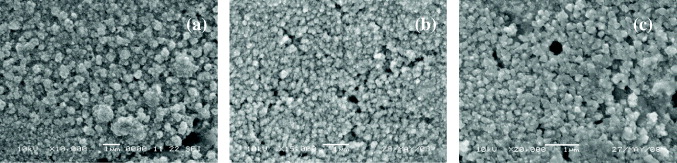
The morphologies of the Ppy films synthesized in different electrolyte solutions were studied by SEM and are shown in figures 3 and 4. Figure 4 shows Ppy films synthesized from Ppy/Cu electrodes with 5 h of drying at 40–45 °C, and figure 3 shows Ppy films synthesized from Ppy/Cu electrodes without heat treatment.
Figure 3 SEM images of Ppy films without heat treatment synthesized in solutions of (a) oxalic acid, (b) oxalate buffer and (c) salicylic acid.
Figure 4 SEM images of Ppy films dried at 40–45 °C for 5 h synthesized in solutions of (a) oxalic acid, (b) oxalate buffer and (c) salicylic acid.
Under non-heat-treatment conditions, the Ppy film formed in oxalate buffer solution (figure 3(b)) was better structured (tiny grains of almost the same size) than that formed in the oxalic acid solution (figure 3(a)). The Ppy film formed in salicylic acid solution (figure 3(c)) has the best structure. This may be caused by the high anodic polarization of the pyrrole polymerization process in the salicylic acid solution (see table 1).
However, with 5 h of drying at 40–45 °C, the Ppy film formed in oxalate buffer solution (figure 4(b)) was better structured than Ppy films formed in either the oxalic acid or the salicylic acid solutions (figures 4(a) and (c)). As shown in figure 4, Ppy films formed in oxalate buffer or salicylic acid solution were nanoporous structures. The grain size (from 100 to 200 nm) of the Ppy film synthesized in oxalate buffer solution is smaller than that (150 to 250 nm) formed in the salicylic acid solution. It can be seen that Ppy films under appropriate heat-treatment condition were much better and had attained a nanoporous structure.
3.2. Nitrate and nitrite electroreduction
3.2.1. Nitrate and nitrite electroreduction on Cu, Ppy/Cu-OA and Ppy/Cu-SA electrodes
Nitrate electroreduction efficiency using Cu, Ppy/Cu-OA, Ppy/Cu-SA, Ppy/Cu-OB and Ppy/Cu-SB electrodes are shown in table 2. The results were obtained after different electrolysis durations at −1.05 V cathodic potential. After 3 h of electrolysis, Cu electrode has shown rather high conversion efficiency for nitrate electroreduction, but formed much nitrite compared to Ppy/Cu-OA and Ppy/Cu-SA electrodes. Nitrite formation in the nitrate electroreduction using Ppy/Cu-OA was low (2.5 times lower than Cu electrode) as well as nitrate electroreduction efficiency (7.3 times lower than Cu electrode).
Table 2. Nitrate electroreduction efficiency and formation of nitrite on Cu, Ppy/Cu-OA and Ppy/Cu-SA electrodes for 3 h of electrolysis.
| Cu | 40.1 | 26 | 64.6 |
| Ppy/Cu-OA | 5.5 | 6.6 | 11.6 |
| Ppy/Cu-SA | 14.4 | 4.2 | 29.3 |
However, nitrate electroreduction efficiency on Ppy/Cu-SA was higher than Ppy/Cu-OA (about 2.6 times) but 2.8 times lower than Cu electrode. On the other hand, nitrite formation from nitrate electroreduction on Ppy/Cu-SA was lower than both Cu (4.3 times lower) and Ppy/Cu-OA electrodes (1.6 times).
3.2.2. Nitrate and nitrite electroreduction of Ppy/Cu-OB and Ppy/Cu-SB electrodes
Nitrate and nitrite electroreduction were carried out on Ppy/Cu electrodes synthesized in oxalate buffer and salicylate buffer solutions for 20 h of electrolysis (table 3). In both Ppy/Cu electrodes, although the nitrate electroreduction efficiency was not as high as for the Cu electrode, nitrite formation was at very low rate (1.9% and 2.1%).
Table 3. Nitrate electroreduction efficiency and formation of nitrite on Ppy/Cu-OB and Ppy/Cu-SB electrodes for 20 h of electrolysis.
| Ppy/Cu-OB | 37.8 | 1.3 | 2.1 |
| Ppy/Cu-SB | 35.8 | 1.2 | 1.9 |
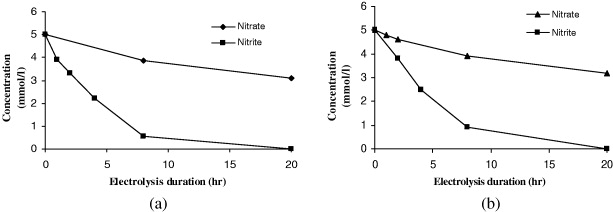
As shown in figure 5(a), the nitrate and nitrite concentrations were decreasing during the electrolysis time. As can be seen, the catalytic activity of Ppy/Cu-OB to nitrite electroreduction is stronger than that of nitrate electroreduction since there is significant slope declination. As a result, after 20 h of electrolysis, the nitrate was reduced to 37.8% efficiency but the nitrite was reduced to 88.8% efficiency after 8 h of electrolysis. Similar to Ppy/Cu-OB, the catalytic activity of Ppy/Cu-SB to nitrite electroreduction is much stronger than the nitrate electroreduction (figure 5(b)). After 20 h of electrolysis, the nitrate was reduced to 35.8% efficiency while the nitrite was reduced to 82% efficiency after 8 h of electrolysis. This was a considerable yield compared to results from the literature, such as 24% efficiency of nitrate electroreduction for 24 h of electrolysis and 86% efficiency of nitrite electroreduction for 8 h of electrolysis using an iridium-modified graphite electrode [25].
Figure 5 Nitrate and nitrite concentrations versus electrolysis duration using (a) Ppy/Cu-OB and (b) Ppy/Cu-SB electrodes.
However, the catalytic activity of the Ppy/Cu-SB electrode in nitrate and nitrite electroreduction is maintained after 50 h of electrolysis while the Ppy/Cu-OA electrode only kept its catalytic activity for 5 h of electrolysis. The characteristics of the Ppy/Cu electrodes prepared in salicylate buffer solution were much better than of those prepared in oxalic acid solution; specifically, their long-term stability was found to last about 10 times longer than that of the latter.
Nitrite formed in nitrate electroreduction on a Ppy/Cu-SB electrode was 10 times lower than nitrite formed from nitrate electroreduction on a Ppy/Cu-SA electrode—16 times lower than on a Ppy/Cu-OA electrode and 43 times lower than on a Cu electrode.
4. Conclusions
The morphology of Ppy films generated on a Cu surface electrode was confirmed by a SEM study of a non-Ppy oxidized Cu surface and a Ppy film structure. The observed difference in morphologies of Ppy films formed in oxalate buffer and salicylate buffer solutions might be due to the difference in oxidized Cu surfaces in those electrolyte solutions. The effect of heat-treatment conditions was also confirmed by a SEM study of Ppy films synthesized in different electrolyte solutions. It can be concluded that Ppy films under adequate heat-treatment conditions was much better and they attained a nanoporous structure in oxalate buffer and salicylic acid solutions.
Electroreduction of nitrate on a synthesized Ppy/Cu electrode obtained a lower efficiency but formed a small amount of nitrite compared to nitrate electroreduction efficiency on a Cu electrode. In particular, the amount of nitrite formed on the Ppy/Cu synthesized in oxalate and salicylate buffer was ten times lower than on the Cu electrode. Electroreduction of nitrite has no efficiency on the Cu electrode but by using a synthesized Ppy/Cu electrode, nitrite was reduced to 80% and 100% efficiency for 8 h and 20 h of electrolysis, respectively.
Acknowledgment
This work was financially supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 140.03.120.09.
Footnotes
Report submitted to the 5th International Workshop on Advanced Materials Science and Nanotechnology IWAMSN, Hanoi, 9–12 November 2010.