Abstract
Nanoparticles (NPs) have been used as drug delivery systems (DDS) exhibiting high cell penetration power. As an antitumor photosensitizer, zinc(II) phthalocyanine (ZnPc) was applied in photodynamic therapy (PDT) since its phototoxic activity promotes death of tumor cells in the presence of laser light. Since drugs do not interact only with tumor cells in living organisms, this study aimed to analyze the action of ZnPc-loaded in nanoparticles (ZnPc-NPs) and in solution (free ZnPc) using peritoneal macrophages as a model of non-neoplastic cells that inhabit the tumoral stroma. NPs were produced by emulsion and evaporation of solvent and characterized by dynamic light scattering and transmission electron microscopy. Assays as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, light microscopy and laser scanning confocal microscopy were performed to evaluate the drug effects in the presence or absence of laser light applied in PDT. NPs exhibited dimensions between 290 and 350 nm and rounded shape. Empty NP did not affect cell viability, showing that these nanocarriers are biocompatible DDS. Free ZnPc was randomly distributed in the cytoplasm, while ZnPc-NP was preferably located near the nucleus. At 5 μg ml−1, free ZnPc caused greater loss of cell viability in the absence of laser when compared to ZnPc-NPs, in the presence or absence of irradiation. In contrast, free ZnPc and ZnPc-NPs (0.5 μg ml−1) promoted cell death to the same extent in cells treated with laser light or not. This demonstrates that the performance of this drug is dose dependent in its free form, but not in its nanoencapsulated form. Cells irradiated with laser (100 mW) and treated with free ZnPc or with ZnPc-NPs showed morphological changes. These observations show that both free ZnPc and ZnPc-NPs irradiated with laser light cause cell damage in peritoneal macrophages.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoparticles (NPs) produced by molecular engineering have been used in several areas such as biology, material engineering, medicine and pharmacy. They show a wide spectrum of properties that distinguish them from bulk materials, basically by virtue of their size, like chemical reactivity, energy absorption and biological mobility [1].
Zinc phthalocyanine (ZnPc) has been used both in vitro and in vivo as an antitumor agent in association with photodynamic therapy. This therapy is based on irradiation of visible light upon tumoral tissues pre-treated with drugs those absorb this radiation, inducing changes in cell redox balance. ZnPc shows photosensitizing activity by generating the production of singlet oxygen in the tumoral area, revealing cytotoxic effect on neoplastic lineages. However, ZnPc needs to be embedded in a suitable delivery system for intravenous administration due to its hydrophobic character [2]. Thus, polymeric NPs have been used to overcome the insolubility of ZnPc, improving its bioavailability and tumor targeting.
Camerin et al [3] used zinc(II)-phthalocyanine disulfide encapsulated into AuNPs as photosensitizer for amelanotic melanoma treatment in C57 mice. They observed that the photosensitizer associated with NP is more easily captured by all tissues when compared to its free form. Furthermore, tumors treated with nanoencapsulated drugs revealed a more efficient response than those treated with free ZnPc.
Polycaprolactone nanoparticles (PCL-NPs) containing ZnPc reduced 97% of A549 cell (lung cancer) viability. This interaction caused morphological changes suggestive of cell death by apoptosis and/or necrosis [4].
Nevertheless, in living organisms, several normal cells coexist in the microenvironment of the tumor parenchyma. Therefore, these cells might be affected by free or nanoencapsulated antitumoral drugs. Nowicki et al [5] suggested that macrophages may stimulate the formation and vascularization of the tumor stroma. Mantovani et al [6] proposed the 'macrophage balance hypothesis', in which these cells may stimulate or inhibit the tumor progression. These findings led us to study the effects of free or nanoencapsulated antitumoral drugs on normal cells present in the tumor parenchyma, such as macrophages.
The present study aimed to analyze the effects of ZnPc in both free and nanoencapsulated forms. To this end, peritoneal macrophages from Swiss mice were used as non-neoplastic cell model. The toxicity of empty PCL-NPs was also evaluated in order to verify if the polymer itself induces cellular damage.
2. Material and methods
2.1. Nanoparticles preparation
Empty NPs and ZnPc-loaded NPs were prepared by the emulsification and solvent evaporation method as previously described [4]. Purified nanoparticles were kept at room temperature and protected from light. Since cells were cultivated in mixture of RPMI medium and GlutaMAXTM-I culture medium (Gibco, USA), the dispersions of ZnPc-loaded NPs and free ZnPc were diluted in this medium to the final concentrations of 0.5 and 5 μg ml−1 of ZnPc. A suspension of empty nanoparticles in culture medium was used as negative control for photobiological activity.
2.2. Nanoparticles characterization
Empty NPs or ZnPc-loaded NPs were diluted in ultrapure water and characterized by dynamic light scattering (DLS) by Zetasizer (Malvern, England) at 25 °C. The nanocarriers morphology was assessed by transmission electron microscopy (TEM) (Morgagni 268 D, FEI, USA). Empty NPs and ZnPc-loaded NPs were diluted in distilled water (1:5). Then, 5 μl of each solution were added to a grid coated with formvar film (Ted Pella, Inc., USA, 0.3% w/v), followed by 5 μl of uranyl acetate 2.5% w/v (BDH Limited, England), and then examined by TEM.
2.3. Peritoneal lavage and peritoneal macrophage culture
Macrophages were obtained from peritoneal lavage using Swiss mice (Mus musculus) from Central Vivarium of Centro de Ciências da Saúde (CCS-UFRJ) and from the vivarium of Laboratório de Proliferação e Diferenciação Celular of Instituto de Ciências Biomédicas (ICB-UFRJ). Two to three months old male and female mice, weighing about 25 g, were used. The animals were euthanized via an overdose of ether inhalation (Aldrich, USA) for 10 min Afterwards, 5 ml of balanced salt solution HBSS (Gibco, USA) were injected into the peritoneal cavity in order to obtain the peritoneal lavage. The liquid containing peritoneal cells was centrifuged (centrifuge 5804R Eppendorf, Germany) at 2600 rpm for 10 min; the supernatant was discarded and the pellet was resuspended with 15 ml of RPMI + GlutaMAX™-I. The cells were counted using Neubauer chamber (Spencer®) and then plated at 2 × 105 (96-well plates) or 5 × 105 (24-well plates) macrophage/well in medium RPMI + GlutaMAX™-I supplemented with gentamycin (Sigma, USA, 25 μg ml−1), penicillin (Sigma, USA, 10 000 U ml−1), streptomycin (Sigma, USA, 10 mg ml−1) and 10% fetal bovine serum (FBS) (Gibco, USA). Plates were maintained overnight before the experiments in oven (Forma Scientific, USA) with an atmosphere of 5% CO2, at 37 °C. The entire experiment was approved by the Ethic Committee of Animal Use in Scientific Experimentation of Centro de Ciências da Saúde of UFRJ (CEUA-UFRJ, n° DAHEICB068).
2.4. Analysis of cytotoxic and phototoxic activities of empty NP, ZnPc-NP and free ZnPc
In the day after peritoneal lavage, macrophages were divided into two groups: (1) non irradiated cells and (2) cells irradiated with laser light at 5 J cm−2 during 14 s (potency of 100 mW). Cells in group 2 were treated with free ZnPc or ZnPc-NPs during 15 min before irradiation. Both groups were submitted to the following conditions: empty NPs, ZnPc-NPs (0.5 μg ml−1 ZnPc), ZnPc-NPs (5 μg ml−1 ZnPc), free ZnPc (0.5 μg ml−1), free ZnPc (5 μg ml−1) or dimethyl sulfoxide (DMSO) (1%). The final volume was 100 μl/well (at 96-well plates) and 250 μl/well (at 24-well plates). After 15 min of interaction, the plates were washed with RPMI + GlutaMAX™-I to remove non-internalized NP or ZnPc. The Photon Laser I (DMC, USA), emitting laser light in the visible red (660 nm), with 100 mW power output was used. Since up to 1% v/v DMSO is toxic to various cell types [7], dilutions of free ZnPc at 0.5 and at 5 mg ml−1 in DMSO were standardized so that no more than 0.65% (v/v) of DMSO could be in contact with the peritoneal macrophages.
2.5. Free ZnPc and ZnPc loaded in nanoparticles cellular localization by confocal laser scanning microscopy
ZnPc fluorescence emission remains unchanged after encapsulation into NP [4]. Based on these observations, we carried out confocal laser scanning microscopy (LSM 510 META, Carl Zeiss, Germany and TCS SP5 AOBS, Leica, Germany) to determine free ZnPc and ZnPc-NPs intracellular location, after 15 min of treatment. To this end, nuclei of peritoneal macrophages were stained by Prolong Gold Antifade reagent (Invitrogen, USA) with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI) and the cells were analyzed at 460 nm wavelength. This antifade mounting medium was prepared from a stock solution (5 mg ml−1, 300 nM) diluted in phosphate buffer saline (PBS), according to invitrogen protocol. After treatment, cells were fixed with 250 μl/well using methanol (Reagen, Brazil) for 3 min and then washed with PBS three times, for 10 min each. The coverslips (Knittel glass, Germany) containing post-treatment fixed cells were incubated with DAPI and antifade mounting medium (100 μl) for 1 min, followed by washing with PBS.
2.6. Cell viability assay through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
An MTT solution (Sigma, USA) at 5 mg ml−1 in PBS was applied in 96-well plates after treatments, to a final volume of 20 μl of MTT/well containing 180 μl of RPMI medium. The plates were protected from the light and incubated in oven (5% CO2 atmosphere, 37 °C) for 4 h. After removing MTT-containing medium from the wells, the formazan crystals generated were solubilized with DMSO (100 μl/well) and the absorption was analyzed in a microplate reader (Sunrise Tecan, Switzerland) at 590 nm wavelength. The average absorbance of all treatments was obtained from the average absorbance between 3 treated wells, excluding the absorbance of wells containing only DMSO (blank). The percentage (%) of viable cells was calculated using the average absorbance obtained from 3 wells/treatment and discounting the medium absorbance of 3 blank wells. The ANOVA test with Newman Keuls post-test was applied to evaluate the results.
2.7. Macrophages morphological analysis
After all treatments, peritoneal macrophages adhered to round glass coverslips were fixed with methanol (250 μl/well) for 3 min and then washed with PBS. Cells were incubated for 30 min with giemsa (500 μl/well, Merck, Germany) and then rinsed with distilled water. The coverslips were dehydrated with several ethanol descending concentrations (Isofar, Brazil), then they were clarified with xylol (Isofar, Brazil) and mounted over slides using Entellan® (Merck, Germany). The slides were analyzed in a light microscope (DM 750 Leica, Germany). The images were registered with a camera (DFC 425, Leica) attached to the microscope.
3. Results
3.1. Nanoparticle size
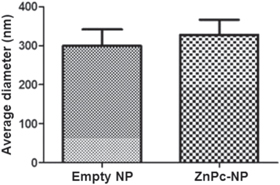
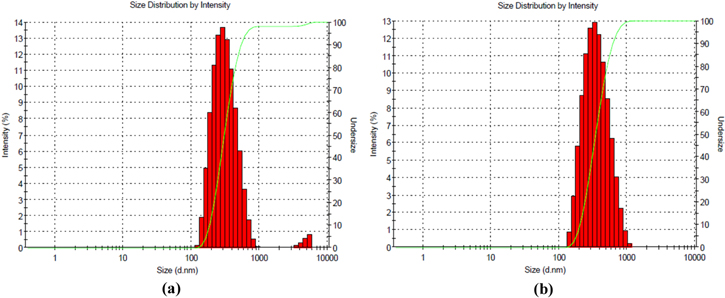
The average diameters and polydispersity index (PI) of NPs are shown in table 1. The PI of empty nanoparticles and ZnPc-NP was around 0.1, indicating their homogeneous size distribution, since this PI value is indicative of monomodal distribution of the sample. The average diameters of NPs were statistically compared using the t-test. No significant difference (p > 0.05) between the diameters of empty nanoparticles and ZnPc-NPs were detected (figure 1). The average sizes of empty NPs or ZnPc-NPs (5 μg ml−1) are shown in figure 2.
Table 1. Average diameter of empty NPs or ZnPc-NPs and polydispersity index.
| Nanoparticles | Average diameter (nm) | Polydispersity index |
|---|---|---|
| Empty | 299.4 ± 42.2 | 0.178 |
| ZnPc-NPs (5 μg ml−1) | 328.3 ± 38.4 | 0.147 |
Figure 1. Diameters of empty NPs and ZnPc-NPs. Drug encapsulation did not significantly change the size of NPs (p > 0.05).
Download figure:
Standard image High-resolution imageFigure 2. Size distribution of polycaprolactone nanoparticles: (a) empty NPs (mean diameter = 299.4 ± 42.2 nm, PI = 0.178), and (b) ZnPc-NPs 5 μg ml−1 (mean diameter = 328.3 ± 38.4 nm, PI = 0.147).
Download figure:
Standard image High-resolution image3.2. Nanoparticles morphology
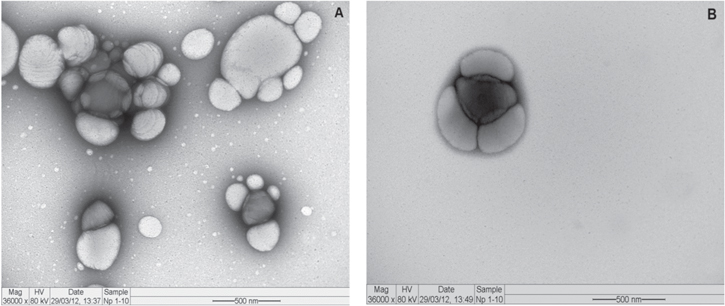
Electron micrographs of empty NPs and ZnPc-NPs (5 μg ml−1) are seen in figure 3. The NPs exhibited variable size and shape (figure 3(A)). Empty NPs had average diameter of 290 nm, corresponding to the average size determined by DLS. Some of these NPs revealed smooth and uniform surface, while others exhibited regular roughness. ZnPc-NPs (figure 3(B)) showed smoother and more homogeneous surface when compared to the empty NPs and their average diameter was 380 nm, similar to that obtained through DLS. In addition, the rounded shape prevailed in both empty NPs and ZnPc-NPs.
Figure 3. Electron micrograph of polycaprolactone nanoparticles. NPs differ in size, shape and surface texture: (A) empty NPs and (B) NPs containing ZnPc (5 μg ml−1).
Download figure:
Standard image High-resolution image3.3. Free ZnPc and ZnPc-NPs cell localization
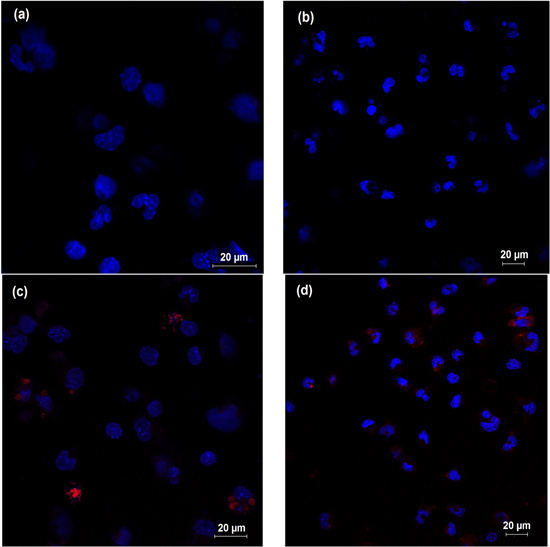
NPs were visualized inside the cells, suggesting that they pass through the cell membrane. Free ZnPc (5 μg ml−1) was visualized to randomly disperse inside and outside the cells (figure 4(d)). On the other hand, the photosensitizer loaded in nanoparticles (5 μg ml−1) predominated in nuclear and perinuclear regions (figure 4(c). The interaction among ZnPc loaded in nanoparticles and macrophages can be seen in video 2. Video 1 shows untreated macrophages (supplementary material).
Figure 4. Cellular location of free ZnPc and ZnPc-NPs (5 μg ml−1) (red dots) near macrophage nuclei (blue dots): (a) untreated cells, (b) macrophages treated with empty NP, (c) ZnPc-NPs predominate around the nucleus in contrast to the random dispersion of free ZnPc (d).
Download figure:
Standard image High-resolution image3.4. Cell viability alterations
Laser light (fluency of 5 J cm−2) caused no significant decrease in macrophage viability. Free ZnPc and ZnPc-NPs toxicities are distinct, considering the applied concentrations. Cell viability reduced about 30% after treatment with free ZnPc or with ZnPc-NPs (0.5 μg ml−1), in non-irradiated cells. Nevertheless, non-irradiated cell viability decreased around 78% after treatment with free ZnPc (5 μg ml−1). These results demonstrate that the cytotoxic action of free ZnPc is dose-dependent, unlike ZnPc-NPs citoxicity (figure 5).
Figure 5. Cell viability. Cytotoxicity and phototoxicity of 0.5 and 5 μg ml−1 free ZnPc and ZnPc-NPs. At 5 μg ml−1, in the absence of laser, ZnPc is more harmful compared to other doses. No significant differences were observed among markers indicated with the same color (p > 0.05).
Download figure:
Standard image High-resolution image3.5. Morphological changes
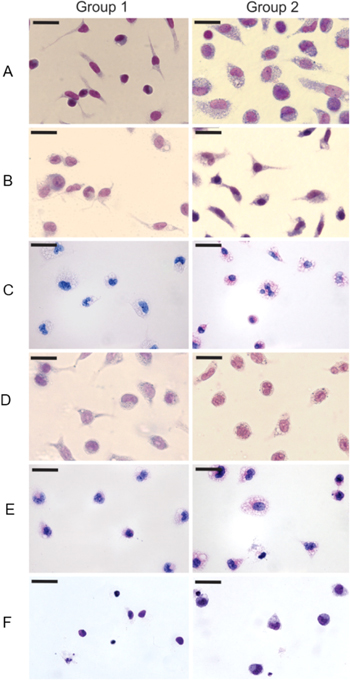
Due to the nanometer dimension, NPs are not visible by light microscopy. However, their cellular morphological effects are visualized through optical microscopy (figure 6). Untreated and non-irradiated macrophages exhibited their normal morphology, i.e., oval nucleus and evident membrane extensions (figure 6(A), group 1). On the other hand, untreated macrophages and irradiated with laser (5 J cm−2) exhibited intense cytoplasmic vacuolization and round nuclei (figure 6(A), group 2), suggesting that morphological changes were induced by laser irradiation.
Figure 6. Macrophages treated with different concentrations of free ZnPc or ZnPc-NPs, with (group 2, 5 J cm−2) or without irradiation (group 1). Note reduction of cell number and morphological changes such as cytoplasmic vacuolization and retraction of cellular projections. The bar on each image has 20 μm size.
Download figure:
Standard image High-resolution imageDespite laser irradiation, macrophages treated with empty NPs showed similar morphology than the control cells, demonstrating that these NPs caused no cell damage (figure 6(B), groups 1 and 2). Most cells treated with ZnPc-NPs (0.5 μg ml−1) displayed loss or thinning of membrane projections, becoming rounded with cytoplasmic vacuoles (figure 6(C), group 1). Such alterations were more evident in cells exposed to ZnPc-NPs (0.5 μg ml−1) and irradiated with laser light (figure 6(C), group 2). Similar changes were visualized in cells treated with 5 μg ml−1 ZnPc-NPs (figure 6(D), group 1), which became spherical and extremely vacuolated. In addition, vacuoles were more prominent in cells irradiated with laser (figure 6(D), group 2). The assays demonstrated that laser irradiation intensifies the morphological changes induced by ZnPc-NPs treatments.
Cells treated with 0.5 μg ml−1 (figure 6(E), groups 1 and 2) and 5 μg ml−1 (figure 6(F), groups 1 and 2) free ZnPc, with or without irradiation, reduced in number and showed injured structures, such as absence or thinning of membrane projections and excessive cytoplasmic vacuolization.
4. Discussion
In this study the average diameter of empty nanoparticles and ZnPc-NPs were 299.4 nm and 328.3 nm, respectively, having no significant statistical difference. NPs with or without ZnPc were round as already described in [8, 9]. The results indicate that the used NPs are stable structures, since the encapsulation induced no significant modification of their average diameter and morphology.
Since ZnPc induces the generation of singlet oxygen, which migrates less than 0.02 μM from its production site, the intracellular location of ZnPc may influence its toxicity [10]. In this study, ZnPc-NPs located close to the macrophage nuclei, indicating that the perinuclear area is their preferential site.
However, free ZnPc was randomly distributed within and among macrophages. Rück et al [11] and Fabris et al [12] observed that ZnPc exhibits random spreading. Our results suggest that nanoencapsulation may target the photosensitizer to a specific region inside cells, representing an advantage of nanoencapsulation since Gomes et al [13] noted that free ZnPc cause side effects, distributed indiscriminately throughout the body.
Empty NPs induced neither significant morphological alterations nor loss of macrophages viability, being biocompatible nanostructures. Therefore, they can be considered promising drug delivery systems, as suggested in [14].
Depending on cell type (or tissue) and irradiation fluency, the laser light individually possesses ambiguous nature, causing damage and loss of cell viability or inducing beneficial effects such as mitosis and tissue regeneration [15–17]. In this study, no significant changes on macrophage viability after laser irradiation were detected. However, laser light promoted considerable morphological changes that included cytoplasm vacuolization and nucleus rounding.
Laser light irradiation also did not alter cell viability after treatments with free ZnPc, unlike its nanoencapsulation. While about 65% of macrophages treated with ZnPc-NPs lost their viability, about 95% of these cells were inactivated after interaction with free ZnPc, suggesting that nanoencapsulation might reduce ZnPc toxicity in acute conditions, possibly by slowing its release.
The results showed that ZnPc-NPs toxicity is not dose-dependent, unlike free ZnPc, which revealed dose-dependent toxicity. At 0.5 μg ml−1, irradiation and nanoencapsulation of ZnPc did not modify its toxicity. Nevertheless, at 5 μg ml−1, the irradiation influenced the toxicity of free ZnPc, since the percentage of viable cells was higher when submitted to irradiation than without irradiation. These results conflict with Soares et al [4] and Machado et al [18].
Depending on experimental conditions, such as concentration, cell type, cellular location as well as incubation time, ZnPc does not significantly affect the function of the mitochondrial enzyme NADH dehydrogenase, evaluated through the MTT assay, as reported by Fabris et al [12]. Likewise, in rat lymphoma cell lineage, silicon phthalocyanine (PC4), ZnPc-like material, showed no direct effect over NADH dehydrogenase [19]. These findings may explain the differences detected in cell viability between 0.5 and 5 μg ml−1 free ZnPc treatments. In this manner, mitochondria might be preserved in most treatments, but this does not necessarily mean that other cell regions are not affected by the drug and/or by the irradiation, as seen through the morphological analysis.
Regardless irradiation, treatments with free ZnPc and ZnPc-NPs caused cytoplasmic vacuolization, the loss of membrane extensions and, in some cases, cell rounding and even cell disruption. At the same time, nanoencapsulation may slow down the release of ZnPc, since macrophages treated with free ZnPc exhibited more morphological damage when compared to those treated with ZnPc-NPs. These results are corroborated by our analysis of cell viability and the results of Soares and co-workers [4].
5. Conclusions
The assays performed in this study allowed to conclude that ZnPc-loaded NPs has a specific site of photosensitization near the cell nucleus. This is an advantage of nanoencapsulation compared to the use of free ZnPc. However, empty NP did not significantly alter macrophage viability or morphology, demonstrating that these nanoproducts are biocompatible. Unlike ZnPc-NPs, free ZnPc reveals dose-dependent cytotoxicity, poorly affecting the activity of the mitochondrial enzyme NADH dehydrogenase, but inducing significant changes in cell morphology. In the present conditions, free-ZnPc or ZnPc-NPs represent dangerous photosensitizes even for non-tumoral cells, such as peritoneal macrophages.
Acknowledgments
Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Laboratório de Desenvolvimento Galênico, Laboratório de Patologia da Fundação Oswaldo Cruz.