Abstract
The present study focuses on the biosynthesis of silver nanoparticles (AgNPs) along with its antibacterial and photocatalytic activity. The AgNPs were synthesized using Cordia dichotoma leaf extract and were characterized using UV-vis spectroscopy to determine the formation of AgNPs. FTIR was done to discern biomolecules responsible for reduction and capping of the synthesized nanoparticles. Further, DLS technique was performed to examine its hydrodynamic diameter, followed by SEM, TEM and XRD to determine its size, morphology and crystalline structure. Later, these AgNPs were studied for their potential role in antibacterial activity and photocatalytic degradation of azo dyes such as methylene blue and Congo red.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, nanotechnology has gained major acclaim in different branches of science owing to its multifaceted, beneficial properties including electrical, optical, chemical stability and catalytic activity [1, 2]. The novel properties of nanoparticles are widely deployed for various applications in medicine, cosmetics, biomedical devices and environmental remediation [3]. Amongst the wide range of available nanoparticles, metal nanoparticles are considered to be more promising as they exhibit unique physical, chemical and biological properties [4, 5]. The properties and function of the nanoparticles are size and shape dependent. Consequently, for a better antibacterial and catalytic activity a specific control over the shape and size of the nanoparticles is prerequisite, which could be achieved by employing different synthesis methods, reducing agents and stabilizers [6–10]. Though, there are several chemical and physical approaches available for the synthesis process, the use of chemicals poses hazardous risk to the environment and are relatively more expensive. Thus, a better alternative is required which can be attained by green synthesis. Green synthesis approach is eco-friendly, cost effective and provides single step synthesis of nanoparticles [10–14]. The process of reduction and stabilization of silver ions is done by the combination of phenolics, tannins, terpenoids, proteins and amino acids present in the plant extracts that are environmentally ubiquitous.
Silver nanoparticles (AgNPs) possess the ability to act against both gram positive and gram negative bacteria. These AgNPs have been incorporated as an efficient antibacterial agent in different applications ranging from disinfecting medical instruments to wastewater treatment. Nanoparticles biosynthesized in the range of 5–50 nm using Justicia adhatoda leaf extract showed potent biocidal activity against Pseudomonas aeruginosa (P. aeruginosa) [15]. Recently, other studies done employing Panax ginseng, Momordia charantia, Solanum trilobatum, Calotropis gigantean and fruit extracts of carambola have proved to act against wide range of pathogens [16–20]. Besides, microalgae have also known to play an eminent role in biosynthesis of AgNPs. In a study, Chlorella vulgaris have shown to display biosynthesis of AgNPs and exhibit antibacterial property efficiently [21]. Although, the mechanism of antibacterial effect is still debated, there are many hypothesis put forward. Positive charge of the silver ions is suggested to play a vital role in exhibiting antibacterial activity. Besides its antibacterial efficacy, AgNPs also show catalytic properties in the field of dye detoxification and its removal. Textile and paper industries commonly employ various non-biodegradable dyes which are potentially hazardous and can cause serious ecological problem. Different methods that are commonly practiced for detoxification of dyes are UV-light degradation, carbon sorption, flocculation and redox treatments. However, these techniques are ineffective and demand a better approach (photocatalytic degradation of dyes). Nowadays, biosynthesized nanoparticles are considered to be more advantageous and economical owing to their biocompatibility. In a study Kumar et al [22] showed catalytic degradation of rhodamine dye in the presence of silver and silver chloride nanoparticles biosynthesized using Solidago altissima.
The present work focuses on the biosynthesis of AgNPs using aqueous extract of Cordia dichotoma leaves. The plant Cordia dichotoma is well known for its medicinal properties. The leaves and bark of the plant have been extensively used for the treatment of various diseases such as fever, dyspepsia, leprosy, diarrhoea, gonorrhoea and burning sensation. The leaves are also used against helminthic diseases, as astringent, diuretic, demulcent, and ulcer for cough [23–26]. The, biosynthesized AgNPs are further investigated for its antibacterial activity and photocatalytic detoxification of dyes such as methylene blue (MB) and Congo red (CR).
2. Experimental
2.1. Materials
The chemicals (AgNO3 and kanamycin) were purchased from Central Drug House, India. The leaf samples of Cordia dichotoma were collected from Central University of Rajasthan campus, Ajmer, India. For the preparation of aqueous extract double distilled water was used.
2.2. Preparation of plant extracts
Healthy leaves of Cordia dichotoma were procured from the campus and were surface sterilized using double distilled water. The clean leaves were then shade dried for a period of 10 days. The aqueous extract of leaves was prepared by boiling 10 g of ground samples in 100 ml of distilled water at 60 °C for 20 min. The extract was further filtered through Whatman filter paper No. 1 and stored at 4 °C.
2.3. Biosynthesis of silver nanoparticles
AgNPs were synthesized by dropwise addition of the aqueous plant extract to the silver nitrate solution of known concentration in an Erlenmeyer flask under stirring followed by centrifugation at 10 000 rpm (Hanil Combi 514R table top refrigerated centrifuge) for 10 min to obtain pellet of AgNPs. The obtained nanoparticles were subjected to washing (thrice) with double distilled water and analysed on UV-vis spectrophotometer (Halo DB-20 Dynamica double beam spectrophotometer).
2.4. Optimization studies for the biosynthesis of silver nanoparticles
2.4.1. Time
The time of the reaction process was optimized with different time intervals (10, 20, 40, 60, 80 and 100 min). The reaction was conducted at 10:1 ratio of AgNO3 solution and plant extract. The resulting AgNPs were analysed on UV-vis spectrophotometer.
2.4.2. Ratio of plant extracts and silver nitrate solution
Similarly, the reaction was performed with different volume ratio of leaf extract and silver nitrate solution for its optimization with 2 mM of AgNO3 solution (1:5, 1:6.5 1:10, 1:20, 1:40). The resulting suspension of AgNPs was analysed on UV-vis spectrophotometer.
2.4.3. Concentration of silver nitrate solution
Herein, different concentration of AgNO3 (0.1 mM–2 mM) was used to determine optimum concentration for the reaction. Thereafter, absorbance of the AgNPs suspension was obtained using UV-vis spectrophotometer.
2.4.4. Temperature
The effect of temperature was investigated on the reaction using different temperature ranges (4 °C, 25 °C, 40 °C, 60 °C and 80 °C). The concentration of Cordia dichotoma leaf extract and AgNO3 solution was kept constant followed by analysis of the suspension on UV-vis spectrophotometer.
2.5. Physicochemical characterization
Initially, the synthesis of AgNPs was monitored by using UV-vis spectrophotometer within the wavelength range of 300–700 nm. Further, Fourier transform infrared spectroscopy (FTIR) analysis was performed with the obtained AgNPs dried under vacuum. The samples were ground with KBr pellets before FTIR analysis. Dynamic light scattering (DLS) was employed to determine the hydrodynamic diameter and polydispersity index using Zetasizer Nano ZS (Malvern Instruments, UK) with 5 mW HeNe laser followed by detection of the scattered light at 173° angle. All the analysis was carried out in an automatic mode and the size of particles were obtained as average value of 13 runs. Morphology and size of the nanoparticles was determined by SEM and TEM. X-ray diffraction studies were done in order to determine the crystalline nature of the biosynthesized nanoparticles.
2.6. Antibacterial activity
The antibacterial efficacy of AgNPs was tested against E. coli and P. aeruginosa by performing microbial disc-diffusion assay. Overnight grown bacterial culture was used to streak the agar plate with a density of 105 CFU ml−1. Discs were impregnated with different concentration of AgNPs suspension (5, 10, 20, 50, 100 and 200 μg ml−1) and placed on the plates with positive (Kanamycin, 10 μg/disc) and negative control (distilled water). The microbial culture plates were incubated at 37 °C for 18 to 24 h. Finally, the zone of inhibition was measured and noted as mean ± SD of the duplicate experiment.
2.7. Photocatalysis
The photocatalytic activity of the AgNPs was evaluated by employing methylene blue (10 mg l−1) and Congo red (100 mg l−1) aqueous solution. Thereafter, the experiments (photocatalytic reactions) were conducted outdoor under the sunlight as main energy source. The experiment was set up by preparing a suspension of AgNPs and the respective dye solution. The mixture was kept under stirring for 30 min in dark to bring the AgNPs to constant equilibrium in the mixture. Later, the mixture was kept under sunlight for 5–6 h. The suspension mixture was then measured at regular intervals after centrifugation to ensure photodetoxification of the dye.
2.8. Statistical analysis
All the experiments were done in duplicates, with three separate experiments to demonstrate reproducibility. All the data were presented as mean ± standard deviation (± SD) of all the experiments. Statistical analysis was performed using a Student's t-test. The differences were considered significant for p < 0.05 and p < 0.01 indicative of a very significant difference.
3. Results and discussion
3.1. Biosynthesis of silver nanoparticles
Generally, the reduction and stabilization of the AgNPs are aided by the phytochemicals and phenolics present in the plant extracts. The leaves of Cordia dichotoma is known to possess alkaloids, saponins, flavonoids, terpenes and sterols [27] that could probably aid in the synthesis of AgNPs. However, with 1 mM AgNO3 solution no colour change was observed. Therefore, the experiment was carried out by taking 2 mM solution and the extract was added dropwise to the solution. The AgNPs formation was indicated by the gradual colour change of the solution from light to dark brown (figure 1), and the characteristic surface plasmon resonance (SPR) peak around 430 nm further confirmed the presence of AgNPs in the suspension.
Figure 1. Visual observation of AgNPs synthesis: (A) AgNO3 before addition of leaf extract and (B) after addition of leaf extract depicting synthesis of AgNPs as the colour turns to dark brown in colour.
Download figure:
Standard image High-resolution image3.2. Optimization for the synthesis of silver nanoparticles
Synthesis of small sized monodispersed nanoparticles is generally dictated by the optimum conditions such as time, temperature, concentration of AgNO3 solution and plant extract. Thus, in order to determine the optimum conditions the aforementioned parameters were optimized. Time plays a major factor in the synthesis of nanoparticles. The synthesis of AgNPs was observed after 40 min. The solution turned from light yellow to brown in colour indicating reduction of silver ions. It was also observed that the synthesis of AgNPs increased with increase in time (figure 2(a)). The reaction was performed till 100 min and the AgNPs showed characteristic peak around 430 nm.
Figure 2. UV-vis spectra of biosynthesized AgNPs recorded as a function of (a) time, (b) concentration of AgNO3 solution, (c) volume ratio of AgNO3 solution and concentration of extract and (d) temperature.
Download figure:
Standard image High-resolution imageDifferent concentrations of AgNO3 solution were also optimized for synthesis of AgNPs. Synthesis of AgNPs started at a concentration of 1.5 mM and showed maximum absorbance at highest (2 mM) concentration. The reaction was performed for 100 min and a characteristic SPR band was observed around 430 nm, indicating efficient formation of AgNPs (figure 2(b)). Similarly, different concentrations of extract were optimized with 2 mM silver nitrate solution. From the graph, it is clear that the yield of AgNPs increased when the concentration of the leaf extract was increased.
The optimum volume of AgNO3 solution and extract concentration for AgNPs synthesis was taken to be 10:1, as above this concentration the SPR band exhibited red shift (figure 2(c)). Further, temperature of the reaction was also optimized to obtain maximum production of AgNPs. From figure 2(d) it is clear that production of AgNPs was highest at 60 °C and 80 °C, whereas minimal level of AgNPs formation was observed at 25 °C. Thereafter, further reactions were performed with volume ratio of 10:1 for AgNO3 solution (2 mM) and leaf extract while keeping the reaction temperature as 60 °C for 60/100 min.
3.3. Physicochemical characterisation of silver nanoparticles
UV-vis spectroscopy is widely used for characterizing noble metal nanoparticles exhibiting SPR in the visible range. The SPR property is attributed to the collective oscillation of electrons on the surface of metal nanoparticles excited by external energy source. Thus, the characteristic plasmon peak gives an account of physical nature of the AgNPs. The SPR band mainly depends on the particle size and dielectric medium [28–31]. Generally, a typical sharp band denotes narrow distribution of the AgNPs [31]. The position and number of peaks also varies with the type of nanoparticles, its shape and size. Besides, broadening of the optical spectra denotes broad size distribution and aggregation of nanoparticles, whilst a prominent narrow band indicates monodispersed distribution of the particles [32]. A single SPR band is expected to appear in case of spherical nanoparticles and two or more SPR bands with anisotropic particles [33].
FTIR was performed in order to determine the probable role of biomolecules of leaf extract involved in the reduction of silver ions and capping for AgNPs. The favourable properties could be ascribed to its phytochemical content such as alkaloids, flavonoids, saponins, terpenes and sterols. Leaves are also known to possess quercitin and quercitrin [27].
The FTIR spectra of C. dichotoma leaf extract and AgNPs are shown in figures 3(a) and (b). The FTIR band at 3437 cm−1 indicates OH stretch, depicting the presence of alcohols and phenols. Similarly, bands procured at 2925 cm−1 and 2854 cm−1 also denotes OH stretch of carboxylic acids. The band at 1637 cm−1 and 1459 cm−1 correspond to N-H bend and C-H bend, respectively. From the spectra obtained, it is clearly evident that polyphenols such as flavonoids, tannins, phenolic acids and certain compounds possessing amine groups are responsible for the formation of AgNPs.
Figure 3. FTIR spectra of (a) Cordia dichotoma leaf extract and (b) biosynthesized AgNPs.
Download figure:
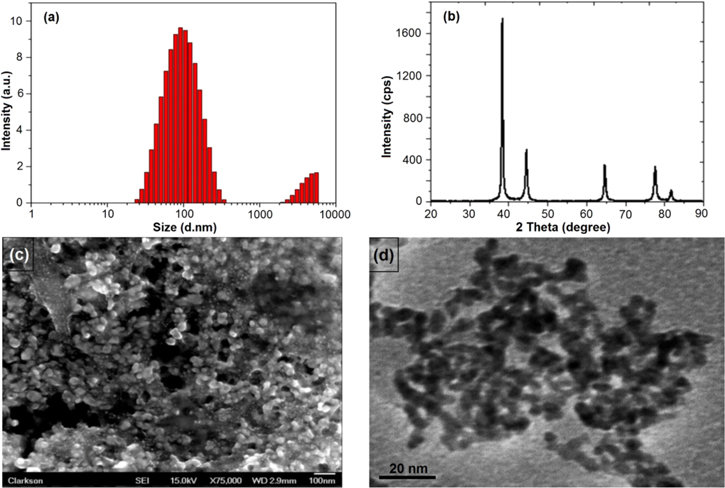
Standard image High-resolution imageDLS technique was used to determine the hydrodynamic size distribution of the particles. In this process, the size is measured by illuminating the particles in Brownian motion by laser beam. The scattered light from the particles is then analysed by the auto-correlator. The mean particle hydrodynamic size of the AgNPs was found to be 90.42 nm with polydispersity index (PDI) 0.3 (figure 4(a)).
Figure 4. Characterization of AgNPs using DLS, XRD, SEM and TEM. (a) Size intensity graph of biosynthesized AgNPs using DLS technique, (b) X-ray diffraction pattern of biosynthesized AgNPs using Cordia dichotoma leaf extract, (c) SEM image of the biosynthesized spherical AgNPs using leaf extract and (d) TEM image of AgNPs.
Download figure:
Standard image High-resolution imageOn the basis of obtained peaks, the crystalline nature of the AgNPs is confirmed. The diffraction peaks 38°, 44°, 64°, 77° and 82° corresponds to (111), (200), (220), (311) and (222) planes of fcc crystal structure that ultimately determines the fcc structure of the AgNPs (figure 4(B)).
SEM was performed in order to determine the size and morphology of the AgNPs. The image showed small sized spherical AgNPs (∼20 nm) uniformly distributed throughout the sample (figure 4(c)). Further, the AgNPs characterized on TEM showed average particle size of ∼10 nm (figure 4(d)).
3.4. Antibacterial activity
The zone of inhibition obtained against E. coli and P. aeruginosa clearly depicts the potent nature of AgNPs synthesized. A considerable difference in the diameter of zone of inhibition was observed with different concentration of AgNPs as displayed in the graph (figure 5). In case of both pathogens it was observed that the AgNPs exhibited its potential activity from a concentration of 10 μg ml−1 up to a concentration of 200 μg ml−1. Several mechanisms of action of AgNPs have been proposed [34–36]. It is noted that AgNPs of 10 nm size or below display activity by itself owing to its smaller particle size and proper cell interaction. Whereas, toxicity caused by 20–80 nm AgNPS is attributed to the release of silver ions inside the cell. Besides, AgNPs react with the thiol groups of proteins and hinders in DNA replication leading to bacterial inactivation [34–36].
Figure 5. Antibacterial activities of AgNPs against P. aeruginosa and E. coli.
Download figure:
Standard image High-resolution image3.5. Photocatalysis
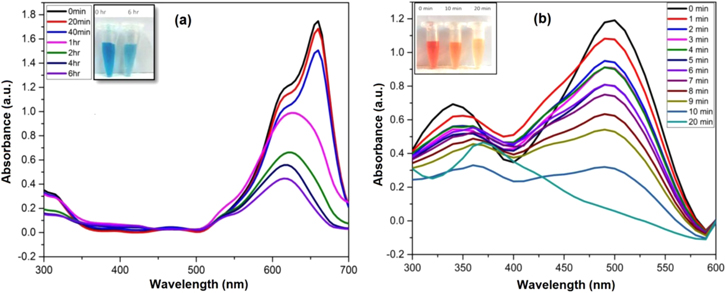
Photocatalytic activity of the AgNPs was determined using detoxification of MB and CR under sunlight for a particular period of time. Initially, the catalytic degradation of the dyes in the presence of AgNPs was observed visually by the change in colour. The intensity of the colour gradually decreased with time from dark blue/orange to light blue/orange marking an effective catalytic degradation activity of AgNPs in the presence of sunlight. Thereafter, the results were confirmed by obtaining absorption band of the dye solution.
MB, an aromatic cationic dye, is widely deployed in textile industries for various purposes. Exposure to the contaminated wastewaters might lead to eye irritation, gastrointestinal tract and skin irritation [37]. Generally, the maximum absorption band of MB aqueous solution is observed at 665 nm owing to the n-π* transition of the MB [38, 39]. The photocatalytic degradation of the MB solution could be determined by the decreasing intensity of the absorption band with respect to time while exposed to sunlight. Thus, SPR property of the AgNPs could be responsible for the decrease in the peak intensity. A similar type of work was performed, where the maximum degradation of MB was attained after 72 h [40]. Whereas in present case, the MB solution was exposed for a period of 6 h that showed significant decrease in the peak intensity (figure 6(a)).
Figure 6. UV-Vis spectra for photocatalytic degradation of (a) methylene blue and (b) Congo red.
Download figure:
Standard image High-resolution imageCR, a secondary diazo anionic dye, is most often used dye in industries. A carcinogenic metabolite, benzidine of CR is known to cause bladder cancer among humans. It is also studied that the effluents with CR are highly coloured and possess high chemical oxygen demand along with high amount of dissolved solids [41]. The photocatalytic degradation of the dye was monitored spectrophotometrically. The characteristic peak of CR is obtained around 500 nm. The degradation of CR dye was confirmed by the gradual decrease of peak intensity at concentration of 0.5 mg ml−1 AgNPs. The complete degradation of dye was observed within 20 min from bright orange colour to light yellow colour (figure 6(b)). The probable mechanism of degradation could be attributed to the SPR effect where the excited surface electrons might interact with the dissolved oxygen molecules and ultimately produce hydroxyl radicals while allowing Ag+ ions to interact with the anionic dye [42, 43]. Hence, it is evident that AgNPs synthesized from Cordia dichotoma leaf extract is highly potential photocatalytic agent for dye degradation in the presence of sunlight.
4. Conclusion
In this study, AgNPs were synthesized using green synthesis approach. The AgNPs were characterized using UV-vis spectroscopy, FT-IR, DLS, SEM, TEM and XRD. Further, the AgNPs exhibited potent antibacterial activity and results obtained were consistent with the results obtained by Kumar et al where the AgNPs displayed its activity at a concentration as low as 5 μg ml−1 [22]. The AgNPs also showed high catalytic activity under the sunlight. These were capable of degrading the dyes such as methylene blue and Congo red. Thus, greener approach for synthesis of nanoparticles could be used as a better antibacterial and catalytic agent.
Acknowledgments
The authors (N G and S N) acknowledge the financial assistance from Science and Engineering Research Board (SERB) (SB/FT/LS-441/2012 and SB/FT/LS-420/2012), Government of India.