Abstract
The solar spectrum consists of 8% UV radiation, while 45% of solar energy is from visible light. It is therefore desirable to fabricate a hybrid material which is able to harvest energy from a wide range of photons from the sun for applications such as solar cells, photovoltaics, and photocatalysis. In this study we report on the fabrication of a TiO2@porphyrin hybrid material by surfactant-assisted co-assembly of monomeric porphyrin molecules with TiO2 nanoparticles. The obtained TiO2@porphyrin composite shows excellent integration of TiO2 particles with diameters of 15–30 nm into aggregated porphyrin nanofibers, which have a width of 70–90 nm and are several µm long. SEM, XPS, XRD, FTIR, UV–Vis and fluorescence spectroscopy were employed to characterize the TiO2@TCPP hybrid material. This material exhibits efficient photocatalytic performance under simulated sunlight, due to synergistic photocatalytic activities of the porphyrin aggregates in visible light and TiO2 particles in the UV region. A plausible mechanism for photocatalytic degradation is also proposed and discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Amongst the many candidates for photocatalysts, anatase (TiO2) is the most promising for industrial use due to its efficient photoactivity, non-toxicity, superior stability and low cost [1, 2]. It is also widely used to both oxidatively or reductively degrade environmental pollutants in air or water in the presence of UV light [3–7]. The commonly reported mechanism upon UV irradiation of TiO2 is the generation of electron–hole (e−/h+) pairs which diffuse to the TiO2 surface to react with OH groups forming OH radicals (OH•). These OH• radicals are considered as the oxidizing agents that degrade the target pollutants [5, 8].
The creation of supramolecular nanoassemblies is a powerful technique for the fabrication of nanostructure materials with tunable morphologies [9–13]. Among numerous organic-based supramolecular nanoassemblies, π–conjugated porphyrins have been attracting great attentions as organic building blocks for the construction of solid-state soft materials [14–19]. Various assembly protocols have been successfully employed to form nanostructured porphyrins, including ionic self-assembly [20], surfactant-assisted self assembly (SAS) [21], reprecipitation [22, 23], etc. Owning to the unique planar geometry, well-understood soret-band in visible region and the inherent capability to mimic biological processes, porphyrin assemblies have many potential applications such as photoelectronics, optical devices, sensors, and solar energy conversion [16, 24–26]. Most recently self-assembled porphyrin nanostructures have been used for visible-light photocatalysis [27–29]. Mandal et al [30] fabricated a range of morphologies of assembled structures (spheres, rods, flakes, and flowers) of meso-tetra (4-carboxyphenyl) porphyrin (TCPP) and used them for the photodegradation of the model pollutant rhodamine B (RhB) under visible light irradiation. Visible light photocatalytic performance of various hierarchically structured porphyrin nanocrystals such as nanosheets, octahedral, and microspheres were also investigated [28].
Conjugated π–electron donor-bridge-acceptor structures of TiO2/porphyrin systems have been extensively studied for various applications such as dye-sensitized solar cells [31–33], and photochemical solar cells [24, 34]. Recently, these dye-sensitized systems have also been considered as an alternative material for visible-light photocatalysis [35]. Shabana et al demonstrated that self assembled monolayers of TCPP on anatase coated-cotton fabric exhibits efficient photocatalytic activity for the degradation of methylene blue and coffee stains [32]. In another study, porphyrin-sensitized TiO2 was successfully studied for photocatalytic degradation of acid chrome blue K [36]. However, both of these studies only investigated the visible light-sensitized property of monomeric porphyrin molecules to enable UV-activated TiO2 photocatalyst having photocatalytic activity under visible light. Literature study clearly revealed that in order to improve the photocatalytic activity of TiO2 under the illumination by sunlight one can either use photocatalyst composites based on TiO2 and carbon nanotubes or apply the plasmonic enhancement effect as recently reviewed in [37, 38].
To the best of our knowledge, this is first example of the fabrication of a TiO2@porphyrin nanocrystal composite for photocatalysis under sunlight irradiation. We have used freebase-tetracarboxy-porphyrin (H2TCP), for fabrication of TiO2@TCPP hybrid materials by direct self assembly of the monomeric TCPP molecules with the assistance of CTAB surfactant and TiO2 anatase nanocrystaline. Typically, TiO2 powder was suspended in a solution of TCPP monomer in NaOH by sonication. The TCPP-adsorbed TiO2 suspension was then added dropwise in a host solution of CTAB in HCl under vigorous stirring to form the TiO2@TCPP aggregates. The photocatalytic performance of the resulting TiO2/TCPP nanofiber hybrid material was evaluated by the degradation of RhB under sunlight conditions.
2. Materials and methods
2.1. Materials
All chemicals were used as received without any further purification. Cetyltrimethylammonium bromide (CTAB), and TiCl4 in HCl were obtained from Sigma Aldrich. Chemicals such as dry acetone, concentrated sulphuric acid (98%), propionic acid, tetrahydrofuran (THF), sodium hydroxide (NaOH), potassium hydroxide (KOH) and ethanol and were purchased from Ajax Finechem.
2.2. Synthesis of H2TCPP
H2TCPP was synthesized following a literature procedure and fully characterized [39].
2.3. Synthesis of TiO2 nanoparticles
A synthetic protocol for preparation of TiO2 anatase nanostructured particles was adopted from previous work [40]. Typically, 3 ml TiCl4 was slowly added dropwise to 30 ml ethanol at room temperature. A light yellow solution was obtained and gelatinized for 2 d to form a sol-gel. Then, the sol-gel solution was dried at 80 °C for 6 h. The dried gel precursor was calcined at 500 °C with a heating rate of 5 °C min−1 for 3 h to obtain the desired TiO2 powder.
2.4. Synthesis of TiO2@TCPP nanofibers hybrid materials
TiO2@TCPP nanofibers were fabricated by a typical surfactant-assisted acid-base neutralization strategy. First, 8 mg of TCPP was dissolved in 1 ml of 0.2 M NaOH solution. Then, 1 mg of TiO2 powder was dispersed in this TCPP solution by sonication for 30 min. This is assigned as the guest solution. The host solution was prepared by dissolving 70 mg CTAB in 19 ml of 0.01 M HCl solution. Subsequently, the guest solution was added dropwise into the host solution under virgorous stirring at room temperature in the dark for 1 h. Free standing TCPP nanorods were also fabricated using a similar strategy without addition of TiO2, for comparative purposes.
2.5. Photocatalytic investigation
Photocatalytic performance of the TiO2@TCPP nanofiber hybrid material was evaluated by the degradation of RhB in aqueous solution. In a typical photodegradation measurement, 0.1 mg of hybrid material was dispersed in a 20 ml aqueous solution of RhB dye with a concentration of 5 mg l−1. The dispersion was stirred in the dark for 30 min to establish an adsorption/desorption equilibrium before irradiation. The sunlight source for the photocatalytic reaction was a 1500 W air cooled xenon lamp. At appointed times, 1.5 ml of dispersion aliquots were taken out and centrifuged to remove photocatalyst. The photocatalytic performance of the as-fabricated samples for RhB degradation was evaluated by recording the real-time absorptivity of RhB at a wavelength of 553 nm.
2.6. Characterizations
The crystal structures and elemental compositions of samples were studied by scanning electron microscopy (SEM) using an FEI Verios 460L (operating under HV and Stage bias condition of 1 keV, using stage bias and circular backscatter detector for low conductive samples). X-ray photoelectron measurements (XPS) were carried out using a K-alpha XPS instrument using monochromatic Al as the x-ray source. The Ti 2p core level spectrum was recorded with an overall resolution of 0.1 eV. The core level spectrum was background corrected using Shirley algorithm and chemically distinct species were resolved using a nonlinear least squares fitting procedure using C as reference. Fourier transform infrared (FTIR) was performed on a PerkinElmer D100spectrometer in attenuated total reflectance mode. A BrukerAXS D8 Discover instrument with a general area detector diffraction system (GADDS) using a Cu Kα source was utilized to obtain XRD patterns. Ultraviolet–visible (UV–Vis) measurements of samples in solution (dissolved in dimethylformamide—DMF) and in solid sate were carried out using a Cary 50 Bio spectrophotometer with a cell of 1 cm path length. A UV–Vis spectrophotometer was also employed to investigate the photocatalytic performance for rodhamine B degration. Fluorescence emission spectra were recorded on a Horiba Jobin Yvon FluoroMax®-4–Spectrofluorometer. All experiments were performed in a quartz cell with a 1 cm path length with a 420 nm excitation wavelength. All the solutions were prepared in a similar manner to that described for the UV–Vis study.
3. Results and discussion
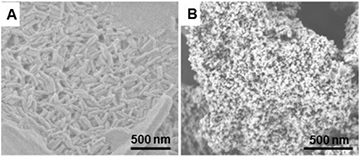
The morphologies of TiO2 nanoparticles, and self-assembled TCPP porphyrin without TiO2 nanoparticles was investigated by SEM as illustrated in figure 1. It can be clearly seen that without TiO2, monomeric TCPP molecules assemble to form nanorod structures 70–90 nm in width and 300–500 nm in length with the assistance of CTAB surfactant (figure 1(A)). SEM image of as-prepared TiO2 is also shown in figure 1(B), which indicates that TiO2 has particle nanostructure morphology with the size distribution in range of 15–30 nm (figure 2).
Figure 1. SEM images of (A) assembled TCPP nanorods without TiO2 powders and (B) TiO2 anatase nanoparticles.
Download figure:
Standard image High-resolution imageFigure 2. Size distribution of prepared TiO2 anatase nanoparticles.
Download figure:
Standard image High-resolution imageIllustrated in figure 3 is the morphology of TiO2@TCPP aggregates obtained from CTAB and TiO2-assisted self-assembly from monomeric TCPP molecules. It clearly shows the formation of aggregated TCPP nanofibers with a width of approximately 100 nm and a length of several µm. Interestingly, as can be seen in the higher resolution SEM and TEM images (figures 3(B) and (D)), TiO2 retains a particle-like nanostructure and is well-integrated into TCPP nanofiber network. The aggregation of porphyrin molecules to form nanofibers with the assistance of TiO2 may be ascribed to the facile absorption capability of carboxyl groups on the porphyrin on the TiO2 surface [34, 41]. Therefore when assembly occurs in the CTAB host solution, TiO2-absorbed TCPP monomers aggregated to form a long fiber structure instead of a rod morphology as is the case for free standing TCPP self-assembly (figure 1(A)).
Figure 3. (A) and (B) SEM images and (C) and (D) TEM images of as-prepared TiO2@TCPP nanofiber hybrid material.
Download figure:
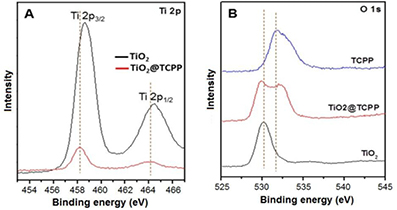
Standard image High-resolution imageThe adsorption of porphyrin onto the TiO2 surface was confirmed by XPS. Figure 4(A) shows the Ti 2p core level of both the TiO2 and TiO2@TCPP composite. The Ti 2p core level of TiO2 has two signals at 458.61 and 464.44 eV, which is attributed to the binding energies of Ti 2p3/2 and Ti 2p1/2, respectively. However, these Ti 2p3/2 and Ti 2p1/2 binding energies in TiO2@TCPP are shifted to lower binding energies at 458.2 and 463.86 eV respectively. These lower binding energy shifts suggest that Ti is accepting electron density from carbonyl groups of the porphyrin, decreasing the binding energy of Ti core level [36]. The adsorption nature of carbonyl-group porphyrin on the TiO2 surface can be ascribed to the weak chemisorptions [42]. The O 1s XPS spectrum of TiO2@TCPP aggregates also indicates the formation of hybrid materials through a chemisorption mechanism (figure 4(B)). The binding energy in the O 1s spectrum of TiO2 shows a main signal at 530 eV, which is consistent with O–Ti–O binding. However, the binding energy of O 1s shifts to lower energy at 529.7 eV after formation of TiO2@TCPP composite, which is in agreement with previous discussion that O–Ti–O group is receiving electron from carbonyl groups of porphyrin, therefore decreasing binding energy of both Ti 2p and O 1s core level. Moreover, by losing electrons in combination with TiO2, the binding energies of O 1s core level of carbonyl groups in porphyrin shift to higher energy as can be clearly seen in figure 4(B).
Figure 4. XPS spectra of (A) Ti 2p and (B) O 1s. The binding energy of Ti 2p core level in TiO2 (black line) and TiO2@TCPP nanofiber hybrid material (red line).
Download figure:
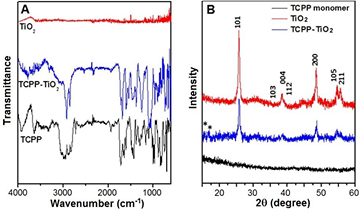
Standard image High-resolution imageThe FTIR spectra of monomeric TCPP molecules, TiO2 nanoparticles and TiO2@TCPP nanofiber hybrid material (figure 5(A)). It is clear that the FTIR spectrum of the hybrid reveals characteristic bands for both TiO2 and porphyrins components. The crystallinity of TiO2, TCPP monomers and the TiO2@TCPP aggregates was determined by XRD as depicted in figure 5(B). The XRD pattern of TCPP monomers (black line) show no diffraction peaks, indicating the non-crystalline nature of monomeric TCPP molecules. Peak positions and widths in the x-ray diffraction (XRD) pattern (red line) of the as-prepared TiO2 particles confirm the synthesis of pure anatase nanocrystalline TiO2 [36]. In the XRD pattern of TiO2@TCPP nanofibers (blue line), apart from peak positions which are well-assigned to anatase TiO2 nanoparticles, the appearance of peaks at about 16° and 17.5° are attributable to the crystalline nature of aggregated TCPP nanofibers, which may due to aromatic π–π stacking between the porphyrin molecules [30].
Figure 5. (A) FTIR spectra and (B) XRD patterns of monomeric TCPP molecules, anatase TiO2 and TiO2@TCPP nanofiber hybrid material.
Download figure:
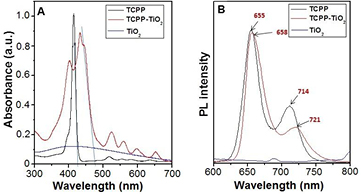
Standard image High-resolution imageOptical properties of the samples were investigated by UV–Vis and fluorescence spectroscopy as shown in figure 6. UV–Vis spectra of monomeric TCPP molecules and the TiO2@TCPP nanofiber hybrid material are shown in figure 6(A). The UV–Vis spectrum of pristine TiO2 nanoparticles shows a broad peaks at around 400 nm. The UV–Vis spectrum of monomeric TCPP molecules shows one strong Soret absorption peak at 416 nm as a result of a transition from a1u(π) to  (π), and four weak peaks in range of from 500–700 nm which are attributed to Q bands of the a2u(π) to
(π), and four weak peaks in range of from 500–700 nm which are attributed to Q bands of the a2u(π) to  (π) transition [43, 44]. After self-assembly with the assistance of the surfactant and TiO2, the UV–Vis spectrum displays characteristic absorption peaks of both TiO2 and porphyrin nanofibers. While the peak at around 400 nm is ascribed to TiO2 nanoparticles, the strong absorption peak at 431 nm with a shoulder at around 445 nm, and four weak peaks in range of 500–700 nm are ascribed to Soret band and Q bands of aggregated TCPP crystals, respectively. In comparison with the Soret band of TCPP monomers, there are distinct bathochromic and hypsochromic shifts by 15 nm from 416 nm to 431 nm indicating that most of the TCPP building blocks form J-aggregates during supramolecular assembly with the assistance of CTAB surfactant and TiO2 [21, 45, 46]. The red-shift in the Q bands also suggest well-defined J-type assemblies of TCPP monomers in conjunction with TiO2 nanostructured particles. The fluorescence properties of the monomeric TCPP molecules and TiO2@TCPP hybrid materials were investigated by photoluminescence spectra (figure 6(B)). The fluorescence spectrum of TCPP molecules in DMF soltuion shows two characteristic emission peaks at 655 and 714 nm. However, in the fluorescence spectrum of TiO2@TCPP, we observed two emission peaks at 658 and 721 nm. These red shifts in the emission peaks are likely due to the coupling from the spatial packing of the TCPP porphyrins [28].
(π) transition [43, 44]. After self-assembly with the assistance of the surfactant and TiO2, the UV–Vis spectrum displays characteristic absorption peaks of both TiO2 and porphyrin nanofibers. While the peak at around 400 nm is ascribed to TiO2 nanoparticles, the strong absorption peak at 431 nm with a shoulder at around 445 nm, and four weak peaks in range of 500–700 nm are ascribed to Soret band and Q bands of aggregated TCPP crystals, respectively. In comparison with the Soret band of TCPP monomers, there are distinct bathochromic and hypsochromic shifts by 15 nm from 416 nm to 431 nm indicating that most of the TCPP building blocks form J-aggregates during supramolecular assembly with the assistance of CTAB surfactant and TiO2 [21, 45, 46]. The red-shift in the Q bands also suggest well-defined J-type assemblies of TCPP monomers in conjunction with TiO2 nanostructured particles. The fluorescence properties of the monomeric TCPP molecules and TiO2@TCPP hybrid materials were investigated by photoluminescence spectra (figure 6(B)). The fluorescence spectrum of TCPP molecules in DMF soltuion shows two characteristic emission peaks at 655 and 714 nm. However, in the fluorescence spectrum of TiO2@TCPP, we observed two emission peaks at 658 and 721 nm. These red shifts in the emission peaks are likely due to the coupling from the spatial packing of the TCPP porphyrins [28].
Figure 6. (A) UV–Vis absorption spectra and (B) photoluminescence spectra of monomeric TCPP molecules (black line) and TiO2@TCPP nanofiber hybrid material (red line).
Download figure:
Standard image High-resolution imageIt is well-known that TiO2 nanoparticles have a band gap energy of 3.2 eV, and have been employed as a photocatalyst under UV irradiation for many applications [47]. Furthermore, porphyrin aggregates have a molecular structure that is comparable to photoactive molecules such as chlorophyll, which show photocatalytic properties in many biological energy transduction processes in plants and algae [48, 49]. The band gap energy of free standing TCPP nanorods and aggregated TCPP nanofibers with TiO2 can be calculated from the UV–Vis spectrum in figure 7(A) to be about 2.88 and 2.55 eV, respectively, which indicates that the photocatalysis can be performed under visible light, and the introduction of TiO2 may tune the band gap energy of the porphyrin aggregates. Based on this calculated band-gap energy, it may suggest that the as-prepared TiO2@TCPP nanofiber hybrid material can be employed as a photocatalyst under sunlight irradiation (both UV and visible lights). Thus, in this work we evaluated the photocatalytic performance of TiO2@TCPP composite in comparison with TiO2 nanoparticles and free standing TCPP nanorods for the degradation of RhB dye under sunlight irradiation. The decrease in the absorption peak of 553 nm of dye as a function of time is monitored to assess the photocatalytic performance. Figure 7(A) shows the C/Co versus time plot of RhB where Co is the initial concentration of RhB and C is the concentration at time t. The plot of ln (At/A0) versus time was also drawn to determine the kinetics of the photocatalytic reaction, where At is the peak intensity at time t, and Au is the intensity at time zero (figure 7(B)). For the blank experiment without photocatalyst, negligible RhB degradation could be observed, suggesting that the self-sensitized photodegradation of RhB does not occur readily under these conditions. When free standing TiO2 nanoparticles and TCPP aggregates are used as photocatalysts, a decrease in RhB concentration of 23% and 50% were observed, respectively. While the decrease in RhB concentration with TiO2 is likely due to photodegradation of RhB molecules under UV irradiation in sunlight, RhB degradation with free standing TCPP aggregates reveal photocatalytic activity under visible irradiation [30]. However the photocatalytic performance increases significantly when the TiO2@TCPP nanofiber composite is used, and RhB degradation reaches 78%, with a rate constant of ca. 7.1 × 10−3 min−1. These results indicate that TiO2@TCPP nanofiber hybrid material can efficiently harvest a wide range of light energies under sunlight conditions for photocatalytic degradation of RhB pollutants.
Figure 7. (A) Photocatalytic performance for RhB degradation and (B) kinetic simulation curve of (a) control without catalyst, (b) TiO2, (c) free standing TCPP nanorods, (d) TiO2@TCPP nanofiber hybrid material.
Download figure:
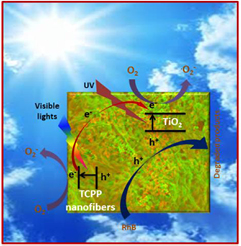
Standard image High-resolution imageIt has been well demonstrated that for the J-type assembly of π–conjugated organic dyes, that electronic delocalization spans over the aggregated molecules as a result of the strong intermolecular π–π interactions [15, 21], which gives J-aggregated porphyrins photosemiconductor properties. Thus, J-type porphyrin assemblies can serve as organic semiconductors, which can harvest light, especially in the visible range, to generate electron-hole pairs under irradiation [15, 46, 50, 51]. It also is known that porphyrin aggregates may increase charge separation due to exciton-coupled charge transfer processes in J-type porphyrin aggregate/TiO2 hybrid materials [33, 52]. This synergistic effect of porphyrin aggregates, along with high photocatalytic performance of TiO2 under UV irradiation could make the TiO2@TCPP nanofiber hybrid material an efficient photocatalyst under sunlight conditions. Based on the well-documented understanding and above discussions, we propose a plausible mechanism for the enhanced photocatalytic activity of the TiO2@TCPP nanofiber hybrid material as shown in figure 8. When TiO2@TCPP nanofibers are irradiated under sunlight conditions, TCPP nanofibers absorb photon energy from visible light to generate e−/h+ pairs, by electrons jumping from the valence band (VB) to the conduction band (CB) [53]. While one portion of generated electrons in TCPP fibers will be transferred to the CB of TiO2 [33] the remaining electrons will participate in the reduction of O2 to. . On the other hand, TiO2 can also harvest light energy in the UV region to generate e−/h+ pairs [54]. These electrons associated with electrons ejected from TCPP nanofibers also reduce the oxygen in H2O. RhB molecules are oxidatively degraded on the surfaces of both TiO2 and TCPP fibers by the holes generated from sunlight illumination of the TiO2@TCPP nanofiber hybrid material.
. On the other hand, TiO2 can also harvest light energy in the UV region to generate e−/h+ pairs [54]. These electrons associated with electrons ejected from TCPP nanofibers also reduce the oxygen in H2O. RhB molecules are oxidatively degraded on the surfaces of both TiO2 and TCPP fibers by the holes generated from sunlight illumination of the TiO2@TCPP nanofiber hybrid material.
Figure 8. The plausible mechanism of GNPs-supported photocatalyst for Rhdamine B degradation.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, we have successfully fabricated a TiO2@TCPP nanofiber hybrid material by CTAB surfactant-assisted co-assembly of monomeric TCPP molecules with TiO2 nanoparticles. The as-prepared TiO2@porphyrin composite shows good integration of TiO2 particles with a diameter of 15–30 nm with aggregated porphyrin nanofiber with a width of 70–90 nm and length of several µm. The TiO2@TCPP hybrid material exhibits efficient photocatalytic performance under sunlight conditions due to synergistic photocatalytic activities of both porphyrin aggregates in visible light and TiO2 particles in UV light. The mechanism for photocatalytic degradation is also proposed and discussed. This work will certainly contribute more insight into the fabrication of hybrid materials, which can harvest a wide range of photon energies from sunlight for photocatalysis, provides a possible solution for environment problems where degradation of organic contaminants in an efficient process is necessary.
Acknowledgments
DDL acknowledges RMIT University and Government of Vietnam under the program 165 for financial support. SVB acknowledges the Australian Research Council under a Future Fellowship Scheme (FT110100152). The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy and Microanalysis Research Facility at RMIT University.