Abstract
In recent years, nanocarriers have emerged as effective platforms for delivering several kinds of herbal medicine and naturally bioactive compounds. In this study we developed an outstanding thermosensitive dendritic nanocarrier to efficiently deliver malloapelta B (Mall B), which is a water insoluble bioactive compound isolated from leaves of Mallotus apelta—Vietnamese medicinal plant. The thermosensitive poly(N-isopropylacrylamide) (PNIPAM) polymer-conjugated polyamidoamine (PAMAM) dendrimer copolymer was prepared via Michael reaction. The copolymer structures were confirmed by proton nuclear magnectic resonance (1H NMR). Morphology of the nanocarrier was observered around 70–120 nm by transmission electron microscopy (TEM). Size distributions were measured by dynamic light scattering (DLS) of the nanocarrier and its Mall B-loaded performed at 146.8 nm and 194.5 nm, respectively. The PNIPAM-g-PAMAM-based nanocarrier exhibited higher Mall B loading efficiency (DL = 59.93 ± 0.19%) and entrapment efficiency (EE = 89.98 ± 2.06%) as compared to PNIPAM (DL = 52.54 ± 0.45% and EE = 66.45 ± 2.78%). In vitro release indicated that approximately 30% amount of the loaded Mall B released at pH 5.5 after 54 h tracking. At the same time, 12.5% amount of the molecules released at pH 7.4.Cytotoxicity assay results showed that the Mall B-loaded nanocarrier significantly inhibited HepG2 cancer cell proliferation. These obtained results indicated that the nanocarrier could solve hydrophobic property of Mall B for further medicine applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The majority of popular anticancer drugs in clinical trials nowadays have limited dissolution and bioabsorption in the human body due to poor water solubility [1–3]. This causes limited transportation in drug delivery, erratic absorption, and bio-avability [4, 5]. Thus, upgrading therapies is a prerequisite to improve the drug delivery and bypass some bottlenecks of bio-related chemotherapy and enhance specific drug release in tumor cells. Broadly, various approaches to the targeted issues with the help of either physical methods such as micromization [6], nanosuspension [7], drug dispersion in carriers [8, 9] or chemical modifications: particularly, salt formation [10], co-solvency [11]. Recently, novel drug delivery systems (NDDS) have sparked a growing interest in improving the therapeutic efficacy of poorly soluble drugs with various researches of NDDS based on nanoparticles [12–14], polymer-drug conjugates [15–17], liposomes [18] or nanocarriers [19–23]. Remarkably, smart polymeric nano-carriers have been explored as 'intelligent' delivery systems able to release, at the appropriate time and site of action, encapsulated drugs in response to specific physiological triggers. Intensively, of all those outstanding polymeric nanocarriers, the temperature responsive was a potential candidate to facilitate the dendrimer with controllable profiles in anti-cancer drug delivery [24, 25].
Along with the expansion of NDDS, there has been a change in human awareness from conventional drugs chemotherapies to the alternative oncology treatments with herbal medicines. Medicinal herbs and their derivative compounds are being increasingly recognized as useful complementary regimens. A large volume of clinical studies have reported the promising impacts of herbal medicines on the survival or immune modulation [26–29]. Furthermore, natural therapeutics from herbal bioactive compounds are more beneficial to the drug commercials due to their localized distributions. Mall B is a particular example which was a bioactive compound isolated from the Vietnamese traditional medicinal plant Mallotus apelta (M. apelta) [30]. It was shown that the nuclear factor-κB (NF-κB) activation suppressed by Mall B led to the down-regulation of the target genes involved in inflammation (TNF-α, IL-6, and IL-1β) and proliferation (iNOS and COX-2) [31]. However, its highly hydrophobic chemical structure resulted in reducing potential in medical application. Markedly, nano-carriers applying to herbal regiments will be expected to carry optimum amount of the drug to their site of action bypassing all the barriers and increase the prolonged circulation of the drug into the blood due to their small size [32–35].
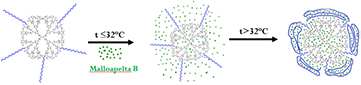
Among nano approaches to overcome the highly water insolubility of Mall B, temperature responsive polymeric dendrimer PNIPAM-g-PAMAM G3.5 was chosen as a promising nanocarrier due to the prior high efficacy results in study of the encapsulation and slow-controlled release (figure 1) [36]. Significantly, the increase in generation numbers of dendrimer not only induced the higher hydrophobic core-shell structure which successfully entrapped much more drug with the same properties but also reduce the amine group on the surface. Moreover, the release of Mall B from PNIPAM-g-PAMAM G3.5 was under the slow-controlled system with the dominance of thermosensitive polymer PNIPAM in the blood heat (above LCST).Thus, the combination of temperature response and high drug inner cavity could bring various high prospects into the future of the smart nanocarrier PNIPAM-g-PAMAM G3.5 in NNDS.
Figure 1. Mechanism of Mall B encapsulated in G3.5-g-PNIPAM.
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Materials
Poly(N-isopropylacrylamide) maleimide and dimethyl formamide were purchased by Sigma. PAMAM dendrimer was synthesized by Department of Materials and Pharmacy Chemistry (Institute of Applied Materials Science) following the Tomalia process [5, 20]. Mall B was isolated from the leaves of M. apelta conducted by Institute of Marine Biochemistry (IMBC-VAST). Regenerated cellulose MWCO 3500–5000 Da and 14 000 Da dialysis bags were from Spectrum Laboratories Inc. All other chemicals and solvent were used without further purification.
2.2. Synthesis of the PNIPAM-g-PAMAM G3.5
PNIPAM-g-PAMAM G3.5 was synthesized via two steps of Michael reaction. Briefly, PAMAM G3.0 (0.045 mmol) and maleimide-terminated PNIPAM (0.315 mmol) were separately dissolved in 20 ml of DMF, and then, PAMAM G3.0 solution was added drop-wise under stirring condition. The reaction was conducted at a room temperature in 48 hours under N2 condition. After that, excess methyl acrylate (0.3 mmol) was slowly added into the mixture to convert all unreactive aminegroups. The reaction was continuously kept at the room temperature stirring condition in 96 hours with N2. Then, it was dialyzed by regenerated cellulose MWCO 12 000–14 000 Da dialysis bags in methanol during 4 d and removed solvents from the sample via rotary evaporation (figure 2).The copolymer was characterized by 1H NMR and TEM, DLS and UV–Vis measurement.
Figure 2. Synthetic scheme of PNIPAM-g-PAMAM G3.5.
Download figure:
Standard image High-resolution imageTo record thermosensitive property of the grafted copolymer, the optical absorbance of PNIPAM and PNIPAM-g-PAMAM derivative solutions in distilled water (1 mg ml−1) at various temperatures was measured at 293 nm photolab®6600 UV–Vis spectrometer. The sample cells were thermo-stated at different temperatures from 30 °C to 35 °C prior to measurements. The LCST values of the sample solutions were defined as the temperature showing an optical transmittance of 50%.
2.3. Encapsulation of malloapelta B in PNIPAM-g-PAMAM G3.5
20 mg of Mall B and PNIPAM-g-PAMAM G3.5 was dissolved in mixture of 20 ml polysorbabe 80 (0.0625%, tween 80) and 20 ml of ethanol then sonicated for 15 min. After that, Mall B-containing solution was vapor rotated and free-dried to attain the Mall B-loaded copolymer. The Mall B-loaded PNIPAM-g-PAMAM G3.5 solution was dispersed again in DI water at 25 °C, warmed up to 37 °C and added into membrane dialysis for indirectly evaluating Mall B loading and releasing efficiencies by UV–Vis method with UV–Vis 6600 spectrophotometer. The evaluation was triplicated. The drug loading (DL %) and entrapment efficiency (EE %) of malloapelta B in thermosensitive dendrimer were calculated from the following equations [36]
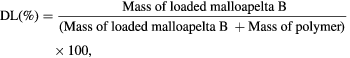
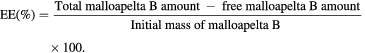
2.4. In vitro drug release
The Mall B-loaded colloidal copolymer solution was added to the dialysis bag 3500 Da and dialyzed with 12 ml of phosphate buffer saline (PBS) solutions at pH 7.4 at 37 °C. At the predetermined time interval, 1 ml of dialyzed solution was drawn to determine Mall B and replaced with an equivalent volume of PBS into each tube. Mall B release was determined by the UV–Vis method with the absorption wavelength at 264 nm.
2.5. Cytotoxictiy assay
The experiment was conducted via sulfordodamine B (SRB) colorimetric assay at Faculty of Biology—University of Science, Vietnam National University in Ho Chi Minh City. Similarly, PNIPAM-g-PAMAM G3.5, free Mall B, and Mall B loaded in PNIPAM-g-PAMAM G3.5 were optimized at the screening to test the inhibition capability of cell growth. The HepG2 cell line preserved in liquid nitrogen was thawed to culture them to 4th generation. Then, HepG2 cells were seeded in 96 well plates (104 cells/well) and allowed 24 h for cell growth 4 in culture medium with 5% CO2 atmosphere at 37 °C. Then, the medium was replaced by the tested samples with a predetermined concentration and incubated for 48 h later. The culture medium were divided into 2 separate medium, which one considered as 'negative control' while another was 'blank sample' containing samples but without cells. After being incubated, the cells were fixed with 50% (wt/vol) trichloroacetic acid and stained with 0.2% (wt/vol) SRB for 20 min. The samples were washed repeatedly (5 times) and clearly with 1% (vol/vol) acetic acid. Finally, the protein-bound dye was dissolved in 10 mM tris-base solution to determine the optical density (OD) with the absorption wavelength at 492 nm and 620 nm. Based on the OD results of control, blank and sample the growth inhibition values were calculated [37, 38].
3. Results and discussions
3.1. Characterization of G3-PNIPAM and PNIPAM-g-PAMAM G3.5
In figure 3 the proton peak of the PNIPAM-g-PAMAM G3.5 copolymer was clearly indicated that there was a co-existence of PAMAM G3.5 with several typical resonance signals such as peak (d) at 3.73–3.78 ppm [–COOCH3], (e) at 3.26–3.36 ppm [–CONHCH2CH2N], (a) at 2.57–2.63 ppm [–CH2CH2N] and the polymer PNIPAM signals as –NHCOCH(CH2)2 (peak (i)), –CH2-(peak (h)), CH(CH3)2NHCO-(peak (l)). Apparently, it was indicated that thermosensitive NiPAM polymer grafted into PAMAM and methylacrylate was reacted to-NH2 groups of PNIPAM-g-PAMAM G3.0. Hence, it was seen that appearance of the conjugated methyl acrylate and PAMAM dendrimer PNIPAM-g-PNIPAM protons.
Figure 3. 1H-NMR spectrum and structure of the PNIPAM-g-PAMAM G3.5.
Download figure:
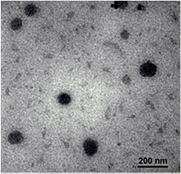
Standard image High-resolution imageFurthermore, morphologies of the thermosensitive nanocarriers were observed by transmission electron microscopy (TEM) as shown in figure 4 with the size of the PNIPAM-g-PAMAM G3.5, in the range of 70–120 nm at 37 °C, which was potential in application of the drug delivery nanocarriers.
Figure 4. TEM image of PNIPAM-g-PAMAM G3.5.
Download figure:
Standard image High-resolution imageRemarkably, dynamic light scattering (DLS) analysis demonstrated that the hydrodynamic size of nanocarriers PNIPAM-g-PAMAM G3.5 was approximately 146.8 nm at 37 °C (figure 5(a)). Besides, the entrapment of drug into nanocarriers could be enhanced with the size up to 194.5 nm with the same temperature (figure 5(b)).
Figure 5. Size distribution of the nanocarriers PNIPAM-g-PAMAM-g-PAMAM G3.5 (a) and drug encapsulated nanocarriers (b) versus light intensity determined by DLS.
Download figure:
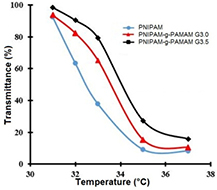
Standard image High-resolution imageIt was shown the temperature made a profound impact on the light transmittance of the PNIPAM-g-PAMAM with different derivatives at 293 nm in distilled water (1 mg mL−1). As in figure 6, the PNIPAM-g-PAMAMs solutions were almost transparent approximately 100% when the temperature was controlled lower 31 °C. However, there was a significant decrease in the transmittance of solutions, in which a transition turned into opacity happened while temperature heated up. In comparison with original PNIPAM, the grafted copolymer showed a higher hydrophilic structure due to graft of PAMAM dendrimer. However, the dendrimer-based copolymer also converted into the micelles at temperature ranging from 34 to 36 °C which could offer a great potential for loading hydrophobic drugs.
Figure 6. Temperature effect on the light transmittance of solutions of PNIPAM-g-PAMAM with different polymers (G3.0 and G3.5) compared to that of PNIPAM.
Download figure:
Standard image High-resolution image3.2. Drug encapsulation and release profile
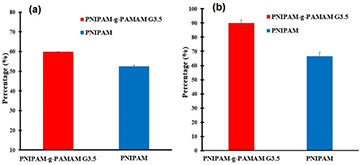
As Mall B being highly hydrophobic active compound, it could not be done in water and thermosensitive PNIPAM-g-PAMAM G3.5 was therefore applied to encapsulation in order to improve its poor solubility. In comparison with thermosensitive PNIPAM both the PNIPAM-g-PAMAM-based nanocarriers exhibited a higher loading (DL = 59.93 ± 0.19%) and entrapment efficiency (EE = 89.98 ± 2.06%) while it was achieved 52.54 ± 0.45% and 66.45 ± 2.78%, respectively in the PNIPAM as shown in figure 7. The results could indicate that interaction of the hydrophobic compound and polymer platform as well a higher cavity space of the dendrimer-based nanocarrier resulting in significantly enhancing drug encapsulation.
Figure 7. Drug loading (a) and encapsulation (b) efficiency of Mall B.
Download figure:
Standard image High-resolution imageMall B was used as a hydrophobic drug model to investigate the release ability of the bioactive compound from the thermosensitive nanocarriers. The drug tracked down from nanocarriers was slower and more stable in the plasma environment (pH 7.4) whilst the PNIPAM-g-PAMAM G3.5 speeded the drug release up in mildly acidic pH = 5.5. In figure 8, a similar phenomenon was happened to the temperature-responsive polymer PNIPAM with a slightly disparity. Under the alkaline (pH 7.4) environment of plasma, the accumulative Mall B release from the dendritic nanocarrier reached at 8.29 ± 0.4% during the first twenty hours, then there was a steady increase in amount of drug into 12.51 ± 0.53% after 54 h. Furthermore, the PNIPAM release trend was under a similarity to PNIPAM-g-PAMAM G3.5 during the same condition, which achieved 11.49 ± 0.93% and 16.68 ± 0.56% of accumulative drug, respectively, after the first day and much more than 2 d.
Figure 8. Drug release profile at pH = 7.4 and pH = 5.5.
Download figure:
Standard image High-resolution imageWhereas, in the acidic (pH = 5.5) environment, PNIPAM and PNIPAM-g-PAMAM G3.5 bore a dissimilarity to those in the neutral. Mall B encapsulated in PNIPAM was achieved the first 24 h with accumulative release rate of approximately 13.33 ± 0.19% then took an upturn to 21.81 ± 0.74% until 54 h, whilst, the PNIPAM-g-PAMAM G3.5 could release 18.53 ± 1.02% and speeded up to 28.71 ± 1.02% of accumulative drug during the similar period. The result is reasonable owning that the nanostructure of thermosensite denrimer nanostructure is swellable due to protonization of amine groups in acidic medium resulting into a higher amount of released drug. Hence, it was promising in the reduction of side-effects of the drug in the blood plasma while facilitating the drug release in the tumor site with acidic medium in control.
3.3. Cytotoxicity assay
HepG2 human liver cancer cell was used to evaluate cytotoxic behavior of thermo-sensitive nanogel PNIPAM-g-PAMAM G3.5, free Mall B and Mall B loaded PNIPAM-g-PAMAM G3.5 using sulforhodamine B dye molecule. The result indicated that PNIPAM-g-PAMAM G3.5 was relatively non-cytotoxic at the experimental condition; merely 6.21 ± 1.87% of the cell was inhibited growth at screening concentration (100 µg ml−1). Whilst, the free drug and drug-encapsulated nanogel exhibited a highly anti-proliferative activity on the cancer cells at the small concentration, in which 50 µg ml−1 of free Mall B reduced 95.57 ± 0.75% of cell growth and 44.75 µg ml−1 of PNIPAM-g-PAMAM G3.5-Mall B induced 97.58 ± 0.83% cell expansion to inhibitory (figure 9).
Figure 9. Anti-proliferative activity of free and Mall B-loaded dendrimer-based nanocarriers against HepG2 cancer cell line at the preliminary screening.
Download figure:
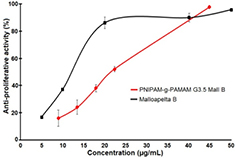
Standard image High-resolution imageMoreover, the free Mall B drug and drug-loaded nanocarriers could inhibit significantly 50% of the HepG2 cancer cell proliferation at 11.32 ± 0.61 µg ml−1 and 20 ± 0.91 µg ml−1, respectively (figure 10). Notably, Mall B-loaded PNIPAM-g-PAMAM G3.5 could exhibit significantly cell growth because amount of Mall B released out of the nanocarrier approximately 20% of loaded amount at point time 48 h (regarding to release profile in figure 8). The result may offers a great potential of the Mall B-loaded PNIPAM-g-PAMAM G3.5 nanocarrierfor inhibiting cancer cell growth in tumor.
Figure 10. Anti-proliferative activity (IC50) of free and Mall B-loaded dendrimer-based nanocarriers against HepG2 cancer cell line.
Download figure:
Standard image High-resolution image4. Conclusion
Thermosensitive PNIPAM-g-PAMAM G3.5 nanocarriers were successfully synthesized and characterized to improve entrapment efficiency of hydrophobic bioactive Mall B as well as to control the slow release drugs delivery systems. Apart from the beneficial of thermosentive polymer to enhance the water insolubility of Mall B, the higher inner cavity of dendrimer-based nanocarrier boosts the loading efficacy. The slow controlled release of drug-loaded nanoplatform and its anti-proliferative ability with cancer cell have brought promising positive impacts to the further dual drug research. These outstanding achievements contribute a pivotal aspect in the development of the effective nanocarriers for delivering several kinds of herbal medicine and naturally bioactive compounds.
Acknowledgment
This research is funded by the Vietnam Academy of Science and Technology in a bilateral project between Vietnam and Bulgaria under Grant number: VAST.HTQT.Bulgaria.01/15-16.