Abstract
This paper highlights the use of the x-ray absorption spectroscopy (XAS) as a local structural tool unlike x-ray diffraction for selected atoms in advanced functional materials including energy storage materials, dielectric materials and thermoelectric materials. The information concerning the oxidation states and local atomic structure around probing atoms will be revealed using x-ray absorption near edge structure (XANES) and extended x-ray absorption fine structure (EXAFS). The XAS beamline: BL5.2 at the Synchrotron Light Research Institute (SLRI) (public organization), Thailand, and its characteristic including available of measured energy ranges, examples of measured spectra of Mg, S and Ti K-edge XAS are also presented. In addition, in situ XAS set up and experiment carried out at this beamline are also outlined.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
These days synchrotron-based x-ray absorption spectroscopy (XAS) is one of the most useful analytical techniques not only for crystalline materials but also for amorphous materials [1–4]. Possibility to verify the existence of fine structures around probing elements in materials is provided by x-ray absorption spectra and the x-ray absorption fine structure (XAFS). Typically, XAS or XAFS refer to the entire spectrum, which comprises the x-ray absorption near edge structure (XANES) which is near the edge spectrum, within 40 eV, and extended x-ray absorption fine structure (EXAFS) to refer to the extended part. Therefore XAS or XAFS became a strong analytical tool for structural studies. Because XAFS does not require long-range crystalline order, it has been used with great success in many research fields, and the basic principles and several applications of the XAFS spectroscopy have been illustrated in many litratures [5–7] and tons of researches especially applications in materials sciences including glasses and liquids [8], catalysts [9, 10], and electrochemical studies including battery studies [11–15].
In more detailed, XAFS is an accurate measure of the x-ray absorption coefficient as a function of incident x-rays, in an energy range, that is, below and above the absorption edge of the selected element of material under investigation. Thus, it is element-specific and comprises electron transition and features that are modified by electron scattering with nearest neighbor atoms. Again, an x-ray absorption spectrum can be divided into two regions which originate with different physical-chemical mechanism: XANES and EXAFS, even though the two structures can be recorded simultaneously in one x-ray absorption spectrum. The XANES region, which comprises the absorption edge and the features immediately beyond the edge (to ~40 eV after the edge), is strongly sensitive to the oxidation states of chemical species and their site symmetry of the absorbing atom. Both quantitative and qualitative information can be obtained from XANES. The latter part called EXAFS region (~1000 eV above the absorption edge) has been used to reveal structural information such as bond distance between interesting atom and its neighbouring atoms and also coordination numbers around that such atoms.
In this paper XAS beamline, BL5.2, at the Synchrotron Light Research Institute (SLRI) (public organization), Thailand, and applications of XAS and in situ XAS on advanced functional materials including energy storages, dielectric materials and thermoelectric materials will be introduced. Finally, concluding remarks on XAS technique will be demonstrated.
2. XAS beamline at SLRI: beamline 5.2
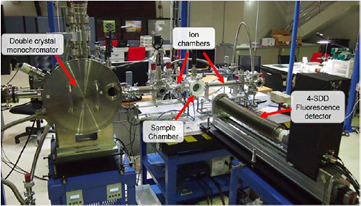
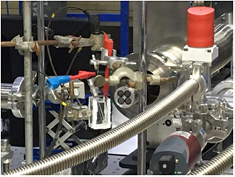
Beamline 5.2: XAS is a flagship beamline at SLRI, Thailand which is constructed under the SUT-NANOTEC-SLRI Joint Research Facility project. This beamline facility is aimed for synchrotron-based XAS research in which the XAS beamtime will be equally allocated to each partner for all service beamtime. The set up of the current BL5.2 SUT-NANOTEC-SLRI XAS beamline is shown in figure 1.
Figure 1. The set up of the current BL5.2 SUT-NANOTEC-SLRI XAS beamline at SLRI, Thailand.
Download figure:
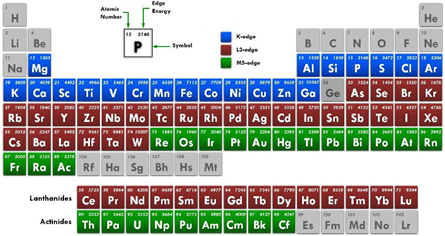
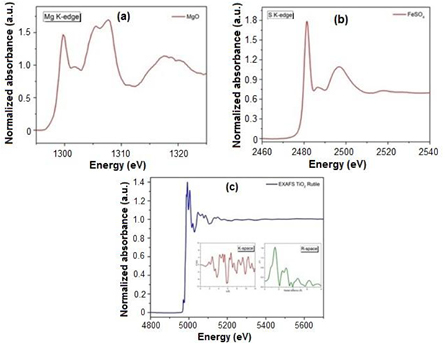
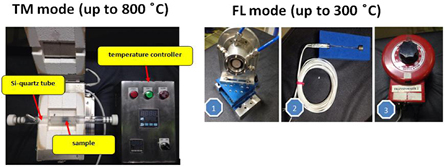
Standard image High-resolution imageThe technical information of this beamline is shown in table 1. The edge energy ranges between Mg K-edge and Ga K-edge (depending on Ga concentration in samples) are available to collect the XAS spectra both transmission mode and fluorescence mode at this beamline. Furthermore, in situ equipments such as XAS heating cells for both transmission mode and fluorescence mode with the reduction and oxidation gas systems (H2, O2 and N2) are also available for the users especially in the catalytic research fields. The edge elements which can be measured (as seen in figure 2), examples of XAS spectra collected at BL5.2 for Mg K-edge, S K-edge and Ti K-edge are also shown in figures 3(a)–(c), respectively. Additionally, XAS heating cells and supported gas systems are shown in figures 4 and 5, respectively.
Table 1. The technical information of BL5.2 SUT-NANOTEC-SLRI XAS beamline.
| X-ray source | Synchrotron: bending magnet |
|---|---|
| Energy range | 1240–121 00 eV |
| Beam size at the sample | 13 mm (width) × 1 mm (height) for transmission mode |
| 20 mm (width) × 1 mm (height) for fluorescence mode | |
| Flux | 108–1010 photons/s/100 mA |
| Energy resolution | 2 × 10−4/light energy |
| Experimental setup | Transmission mode with ion chamber |
| Fluorescence mode with 4-element silicon detector | |
| Crystal type | KTP (0 1 1): 2d spacing (⇒) = 10.955 |
| : energy range = 1250–4780 eV | |
| InSb (1 1 1): 2d spacing (⇒) = 7.481 | |
| : energy range = 1830–7000 eV | |
| Ge (2 2 0): 2d spacing (⇒) = 7.481 | |
| : energy range = 3440–12 100 eV | |
Figure 2. The periodic table of the edge elements which can be measured at BL 5.2, SLRI, Thailand.
Download figure:
Standard image High-resolution imageFigure 3. (a) The Mg K-edge XANES data of MgO sample acquired by KTP (0 1 1) crystal; (b) the S K-edge XANES data of FeSO4 sample acquired by InSb (1 1 1) crystal, and (c) the Ti K-edge EXAFS data of TiO2 rutile sample acquired by Ge (2 2 0) crystal. Converted spectral function of the wavenumber (K-space) and flourier transform (R-space) for analyze atomic structure and local structure of the absorbing atom.
Download figure:
Standard image High-resolution imageFigure 4. In situ XAS heating cells for both transmission mode (left) and fluorescence mode (right).
Download figure:
Standard image High-resolution imageFigure 5. The gas system setup for reduction and oxidation XAS experiments at BL 5.2.
Download figure:
Standard image High-resolution image3. XAS applications on advanced functional materials
3.1. Energy storage materials: in situ XAS study
Pawin et al [16] recently reported a study of the charge storage mechanisms of K-birnassite MnO2 nanosheets and N-doped reduced graphene oxide aerogel (N-rGOae) using an in situ XAS and an electrochemical quart crystal microbalance (EQCM). In their work, the oxidation number of Mn at the MnO2 electrode is found at +3.01 at 0 V versus SCE for the charging process and gets oxidized to +3.12 at +0.8 V versus SCE and then reduced back to +3.01 at 0 V versus SCE for the discharging process. This in situ XAS results of MnO2/N-rGOae supercapacitor may be practically used to improve performance in term of high power and energy applications. Additionally, with a similar in situ XAS study procedure, recent energy storage researches have been carried out in situ XAS study for electrochemical properties in order to reveal a change of charge states of transition metals in samples as reported in [17–19].
Furthermore, Pinit et al [20] and Sukanya et al [21, 22] also reported the studied the fabrication of carbon based metal ferrite composite nanofibers for battery applications. In their works, the studies concerning the structure and electrochemical properties of carbon/MFe2O4 (where M = Mn, Ni and Cu) composite nanofibers produced by a combination of electrospinning and carbonization. XAS is used for this study in order to address an evidence of novel electrochemical properties. From XAS results, structure of MFe2O4 shows cubic inverse spinel ferrite structure with the oxidation states of Fe3+ and Mn3+, Ni2+ and Cu2+ ions in structure, respectively.
3.2. Dielectric materials
Applications of XAS technique in dielectric materials have been recently reported by many research groups [23–28]. One interesting work has been reported by Jakkree et al [23]. In their work, in order to improve the dielectric properties of CaCu3Ti4O12 ceramics, they proposed a co-doping of Sm3+ and Mg2+ in CaCu3Ti4O12 structure. Sm3+ substituted in Ca2+ sites can effectively suppress the grain growth, achieving a fine grained ceramic microstructure. Also, Mg2+ was selected to be substituted into Cu2+ sites to enhance the grain boundary (GB) resistivity for reducing the loss tangent. Effect of co-doping resulted to a slight increase in Ti3+/Ti4+ ratio where this ratio is obtained by the XANES measurement.
Additionally, Jatupol et al [24] also reported a doping of F anions in CaCu3Ti4O12 structure to improve the giant dielectric properties of this material. As obtained from XANES study, effect of F dopant was primarily attributed to the increase in Ti3+ and Cu+ concentrations, due to charge compensation, resulting in a significantly enhanced intensity of space charge polarization at the grain boundaries.
XAS on dielectric materials is also studied from Nuraini et al [25]. In their work, the composition of (1 − x)(K0.5Na0.5)NbO3 − x(Ba0.8Ca0.2)TiO3 and the so-called (1 − x)KNN-xBCT were successfully synthesised using a combination of solid-state reaction and co-precipitation oxalate methods. Local structure distortion due to the dopant was studied by XANES at the Nb L3-edge, XANES at the Ti K-edge, and EXAFS at the Ti K-edge. From EXAFS analysis, the oxygen octahedral distortion and distance of Ti–Nb to its surroundings were observed. These XAS results can address dielectric properties clearly which exhibited in KNN-BCT structure.
3.3. Thermoelectric materials
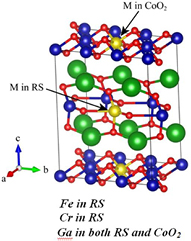
Supree et al [29] and Natkrita et al [30] have recently studied and reported XAS applications on thermoelectric materials. Ca3Co4O9 is one of the most promising thermoelectric oxide materials at high temperature with CoO2 layer and Rock-salt layer (RS). In their studies they reported a structural studies of Ca3Co4−xMxO9 (where M = Fe, Cr, and Ga). The direct evidence for the location of the substitutional elements in the system was revealed using an XAS technique both XANES and EXAFS. The EXAFS spectra were fitted with the models. Also, the XANES spectra was compared with the simulated spectra from the first principle calculation. The data analysis show that for Fe and Cr substitutions, the Fe and Cr atoms are more likely to be located in the RS layer rather than in the CoO2. For the Ga case, Ga atoms accommodate both sites; still the majority stays in the RS layer.
Figure 6. The in situ electrochemical XAS setup in fluorescence mode at BL 5.2.
Download figure:
Standard image High-resolution imageFigure 7. Structure model of [Ca2CoO3]4[CoO2]6 when M is substituted in the RS layer or in the CoO2, adapted from [28].
Download figure:
Standard image High-resolution image4. Conclusion
This paper reports XAS or XAFS including XANES and EXAFS which is certainly one of the most advanced techniques of materials investigation. The characteristic and technical information of beamline 5.2 XAS and its XAS station was reported. Examples of collected XAS spectra at Mg K-edge, S K-edge and Ti K-edge are also presented. These spectra represent a very good quality for low energy XAS beamline. The supported equipments such as in situ XAS heating cell for both transmission and fluorescence-yield modes including a redox gas system are clearly shown and available for users. Last but not least, applications of XAS on advanced functional materials i.e. energy storages, dielectric and thermoelectric materials are reviewed. As obtained from XANES and EXAFS studies, the structural information including valence states and local atomic sites of interesting atoms are pointed out.
Acknowledgments
The author acknowledges Prof Sukit Limpijumnong (SUT), Dr Saroj Rujirawat (SUT), Prof Santi Maensiri (SUT), Assoc. Prof Rattikorn Yimnirun (SUT), Dr Wantana Klysubun (SLRI), Dr Chanapa Kongmark (KU), Dr Kajornsak Faungnawakij (NANOTEC), Dr Gamonwan Tumcharern (NANOTEC), Dr Montree Sawangphruk (VISTEC), Dr Prasit Thongbai (KKU) and Assoc Prof Supree Pinitsoontorn (KKU) for their kind supervision and very great and novel works. Moreover, author would like to thank all XAS users at SLRI, Thailand for their dedicated works. Many thanks to Ms Sompin mahakot, Mr Somboonsup Rodporn, and Mr Dechmongkhon Keawsuwan who assisted in XAS experimental support. Finally, many thank you to the SUT-NANOTEC-SLRI Joint Research Facility for Synchrotron Utilization and the SLRI for synchrotron-XAS facility.
Footnotes
- *
Invited talk at 5th Thailand International Nanotechnology Conference (Nano Thailand-2016), 27–29 November 2016, Nakhon Ratchasima, Thailand.