Abstract
Fungi with metabolic capacities can efficiently synthesize a wide range of nanoparticles (NPs). This biotransformation process and its product have extensive applications especially for industry, agriculture and medicine, where NPs size and shape is essential and can be defined by specific analytical methods. Fungi cultivation and further bioconversion can be fully controlled to obtain the desired nanoparticles. Additionally, this review provides information about the fungus F. oxysporum, which is able to synthesize the largest amount of different types of NPs.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Norio Taniguchi from Tokyo Science University (1974) first simply defined the term nanotechnology as a multidisciplinary science which includes much research and technology [1]. Nanotechnology represents nanoparticles (NPs), (clusters of atoms) ranging in size from 1 –to 100 nm [2]. Examples are titanium [3], magnesium [4], platinum [5], silver [6], gold [7] and silica oxide [8] NPs, which can be produced by myco-fabrication–(NPs synthesis systems using fungi). Apart from fungi, there is a variety of microorganisms producing NPs, such as many bacteria, biofilms [9], actinomycetes [10] and extremophiles [11]. Apart from bacteria, fungi are a favorable option for NPs synthesis. Fungi are filamentous by nature and able to withstand the pressure of flow and mixing in the bioreactor trough. In addition, they can accumulate metals by biological and physicochemical means. Fungi are an excellent choice for large-scale production as biocatalysts because of their ability to secrete extracellular enzymes [7]. By contrast, the bacterial fermentation process involves numerous additional steps to obtain a clear filtrate of colloidal broth [12].
In this review we discuss strategies for NP synthesis with emphasis on the bottom-up myco-approach and its application and flexibility for use in many industrial fields. Our focus is on aspects related to the size of NPs and methods of measuring this feature. It has been discovered that fungi can efficiently synthesize clusters of atoms of a determined size. We also reviewed NPs produced by Fusarium oxysporum (F. oxysporum), which remains the invincible leader in this field.
2. NPs synthesis strategies
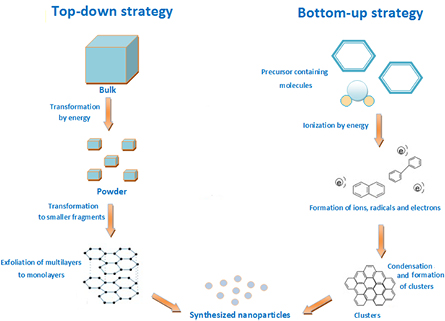
Today, NPs are produced by numerous chemical and physical methods, although more environmentally friendly methods are available. Bottom-up and top-down are two strategies for the synthesis of metal NPs as shown in figure 1. The bottom-up strategy relates to the formation of structures atom by atom, molecule by molecule or by self-organization [13]. Top-down strategy includes the reduction of material to dimensions of nanoscale using cutting, polishing and etching techniques. Such nanomaterials are prepared large scale without inspection at the atomic level [14]. Synthesis of NPs using fungi is one method of bottom-up strategy where a major reaction based on reduction or oxidation of the substrate causes an increase of colloidal structures as was reviewed by Kashyap et al [15]. They proved that fungal enzymes or metabolites are typically accountable for a reduction of metallic compounds in NPs. The way in which myco-reduced metal atoms pass nucleation with subsequent growth and results in the formation of nanostructures is a good example of the bottom-up strategy. The advantage of bottom-up strategy is the preferred feasibility of obtaining nanostructures such as nanorods or nanosheets, without major flaws and more homogenous chemical compositions [15]. Furthermore, bottom-up strategy ensures a greater possibility to receive less defective NPs and much more homogenous chemical composition, due to reduction of Gibb's free energy, by which this strategy is powered [16]. Several attempts were made to obtain specific NPs using both strategies to compare them. One is zirconia NPs, which are appreciated because of their optical and electronic properties for use as materials with electro-optic, dielectric and piezoelectric features [17].
Figure 1. Example of top-down and bottom-up strategies for synthesis of NPs. Based on [18].
Download figure:
Standard image High-resolution image3. Applicability of NPs
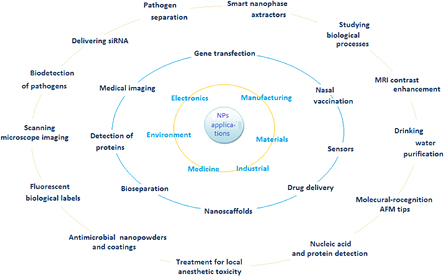
NPs are widely used in many fields, such as electronics, the environmental technology and medicine, where size is essential for manufacturing and materials. Some key examples of applicability are shown in figure 2 [12, 19–21]. Biomedical applications of NPs, such as nanofiber scaffolds, have capabilities to efficiently regenerate the central nervous system as well as other organs. Experiments carried out on hamster with cut optic tracts showed regeneration of nerve tissue launched by a peptide nanofiber scaffold. Management and identification of several diseases might be possible by detecting nucleic acid sequences specific for certain bacteria and viruses, and identifying distinct diseases or unnatural levels of assured proteins that indicate occurrence of numerous diseases, including cancers. Such sensitive methods using NPs can certainly revolutionize the physical treatment of cancer [12].
Download figure:
Standard image High-resolution imageSeveral NP platforms were tested as sensors for detection and separation of pathogens based on their optical and magnetic features. One familiar method used for bacteria detection includes the application of magnetic biosensors which can recognize straight immunological reactions using magnetic NPs coated with antibodies against exterior antigens. This kind of NP's microfluidic appliance was also used to detect bacteria. It was found that core–shell NPs with Fe metal cores have strengthened receptivity opposite to iron oxide nanoparticles (IONPs) for the detection of bacterial cells. This was expanded by a magnetic barcoding approach that did not require antibodies and can recognize single-gene mutations [20].
Due to their properties of high production of specific enzymes, metabolites, rapid growth, easy handling and low cost for large scale production [22], fungi are a desirable material to produce NPs for a defined application. Trichoderma reesei is well known for its extracellular enzyme formation and large quantities of metabolites, requirements that are essential for biosynthesis of silver NPs on a large industrial scale [23]. These silver NPs can be used as efficient growth inhibitors against different microorganisms, such as Escherichia coli, Staphylococcus aureus and yeast, so they can be used for medical devices and antimicrobial control systems [24]. This kind of NPs can be successfully used as nontoxic drugs and genes carriers [25]. Platinum NPs which are synthesized by F. oxysporum in reaction with hexachloroplatinic acid aqueous solution, have anti-cancer and anti-HeLa cells abilities [5, 26].
Suitable applicability of specific NPs depends mainly on their size, and in some cases, shape as well. A few of the methods used to check the size of obtained NPs are described in the following sections.
4. Size of NPs of fungal origin
Synthesis of NPs with various sizes and shapes is essential due to their wide applicability which can be mediated by bacteria, fungi and other organisms. It was found that fungi are almost ideal biocatalysts for NPs biosynthesis, in contrast to bacteria, as they are well-known for producing greater amounts of biologically active substances that make the fungus more appropriate for large-scale production [27]. Furthermore, fungal biomass can resist flow pressure, agitation and harsh conditions in chambers such as bioreactors. They also exude extracellular reductive proteins which can be used in subsequent process steps. Moreover, the cell is deprived of unessential cellular components since NPs are accelerated outside the cell and can be immediately used in manifold ways without pretreatment [28].
There is a large quantity of fungi, which can efficiently synthesize silver NPs, such as Aspergillus clavatus (A. clavatus) [29], Bipolaris nodulosa [30], Cochliobolus lunatus [31] or Trichophyton mentagrophytes [6]. Gold NPs are synthesized using A. clavatus [32], Phanerochaete chrysosporium [33] or Sclerotium rolfsii [34]. The following are all synthesized by F. oxysporum: BaTiO3 NPs [35], CdS NPs [36], Fe3O4 NPs [37], SiO2 NPs [3], Pt NPs [5] and Ti NPs [3]. Mg [4] or ZnO NPs are produced by Aspergillus terrus [38] (table 1).
Table 1. Examples of NPs, their size, types of synthesis (E/I) and their fungal sources.
| NPs | Fungus | Culture medium | Substrate | Substrate concentration (mM) | Size of NPs (nm) | Extracellular (E)/intracellular (I) | References |
|---|---|---|---|---|---|---|---|
| Ag | Aspergillus clavatus | Malt yeast extract agar | AgNO3 solution | 1 | 10–25 | E | [29] |
| Ag | Aspergillus flavus | MGYP | AgNO3 solution | 1 | 17 ± 5.9 | E | [39] |
| Ag | Aspergillus niger | PDB | AgNO3 solution | 1 | 15–20 | E | [40] |
| Ag | Aspergillus terrus | — | AgNO3 solution | — | 1–20 | E | [27] |
| Ag | Bipolaris nodulosa | YM | AgNO3 solution | 1 | 10–60 | E | [30] |
| Ag | Fusarium stolonifer | MGYP | AgNO3 solution | 1 | 5–50 | E | [41] |
| Ag | Lecanicillium lecanii | SMYB | AgNO3 solution | 1 | 45–100 | E | [42] |
| Ag | Neurospora crassa | VMM | AgNO3 solution | 1 | 11 | I/E | [43] |
| Ag | Pestolatia sp. | PDB | AgNO3 solution | 1 | 10–40 | E | [44] |
| Au | Aspergillus clavatus | PDA | HAuCl4 | 1 | 20–35 | I | [32] |
| Au | Phanerochaete chrysosporium | Glucose-maltose | HAuCl4 | 1 | 10–100 | E | [45] |
| 2 | |||||||
| Au | Rhizopus oryzae | PDA | HAuCl4·H2O | — | 10 | E | [46] |
| CdS | Coriolus versicolor (immobilized) | Glucose-maltose | Na2S | 2.5 | 5–9 | E | [45] |
| Cd(NO3)2 | 1.8 | ||||||
| CeO2 | Aspergillus niger | CDB | CeCl3·7H2O | — | 5–20 | E | [47] |
| FeCl3 | Aspergillus oryzae | PDA | FeCl3 solution | 1 | 10–24.6 | E | [48] |
| Mg | Aspergillus terrus | MGYP | MgO | 1 | 48–98 | E | [4] |
| TiO2 | Aspergillus flavus | MGYP | Bulk TiO2 | 1 | 12–15 | E | [49] |
| ZnO | Aspergillus fumigatus | PDA | ZnNO3 | 0.1 | 1.2–6.8 | E | [38] |
In the myco-nanotechnological process of NP synthesis, the fungus mycelium is treated with a metal salt solution which causes the fungi to manufacture their metabolites and activate enzymes to survive. The catalytic effect of these extracellular enzymes and metabolites influences the reduction of toxic metal ions from non-toxic metallic solid NPs. In the case of silver NPs, extracellular fungal reduced nicotinamide adenozin dinucleotide (NADH) and NADH-dependent reductases are responsible for Ag+ to Ag0 reduction [27, 50–54]. It has been shown that the self-assembled protein scaffold controls the size of gold NPs. When the ratio of gold concentration to protein concentration is very high, NPs are not formed and black solid is precipitated [55]. CdS NPs production from Cd2+ and  is caused by fungal reductase enzymes secreted to the environment [36].
is caused by fungal reductase enzymes secreted to the environment [36].
5. Fusarium oxysporum NPs synthesis
F. oxysporum is the leader among organisms producing different types of manufactured NPs. Prefatory analysis of protein associated with the formation of AgNPs by F. oxysporum suggest that reductase dependent on NADH was responsible for this process. This is the reason it was proposed that reductase was responsible for the reduction of silver ions and the succeeding creation of silver NPs, as was mentioned previously [56].
AgNPs synthesis by F. oxysporum has expanded widelyover the last 15 years. In 2002, the fungus was used for intracellular NPs synthesis. Ag+ ions from AgNO3 solution were reduced by this biocatalyst and formed AgNPs from 5 to 15 nm, which were stabilized by fungal secreted proteins. The reaction was performed with 10−3 M Ag+ concentration in 100 ml of distilled water and 10 g of F. oxysporum [57].
In 2005 researchers obtained 20–50 nm AgNPs synthesized by O6 SD, 07 SD, 534, 9114 and 91248 strains of F. oxysporum. Aqueous Ag+ was reduced in the solution and formed silver hydrosol using nitrate-dependent reductase and transfer quinone extracellular process. Other conditions were the same as in the previous example [58]. In 2007, the first photobiological method was used for Ag NPs synthesis that lasted less than an hour, in contrast to dozens of hours spent in previous examples. 10−2 M silver nitrate was added to the supernatant to finally obtain a 10−3 M solution. The synthesis occurred under a halogen-tungsten lamp and provided the opportunity to obtain 5–60 nm NPs [59].
In 2012, F. oxysporum was isolated from soil samples gathered from a mangrove forest in India. 10 g of fungus was added to 100 ml of double distilled water. It was filtered after 48 h and treated with 1mM AgNO3 solution to obtain 5–60 nm AgNPs [60]. From 2013, researchers tried to optimize the process of AgNPs biosynthesis by introducing changes in cultivation media composition, temperature, pH, light intensity, solution concentration, volume of filtrate and biomass quantity.
It was found that application of malt-extractglucose-yeast extract-peptone (MGYP) medium, pH 9–11, 40–60 °C, 190.7 lux and sun light used for NPs producing process made it possible to obtain 21–40 nm AgNPs [61]. It was also reported, that a reaction solution with 1–10 mM AgNO3 as substrate, F. oxysporum as biocatalyst, 560 mM glucose as electron donor, and pH 7 phosphate buffer provides 25–50 nm silver NPs [62].
In 2014 a biotransformation process was carried out by an enzyme isolated from F. oxysporum IRAN 31C, cultured at MGYP, contingent upon reduced nicotinamide adenozin dinucleotide phosphate (NADPH) and gelatin capping agent to avoid aggregation. As a result, a 50 nm AgNPs size was obtained [63].
In 2015 scientists were working on biotransformation parameters which would possibly provide the opportunity to produce the smallest AgNPs synthesized by F. oxysporum. By applying 10−2 M silver nitrate, 11 g of seventh day fungus at 50 °C and pH 6 the researchers could form NPs from 5 to 13 nm [64]. In 2016 researchers have worked on improving the antifungal action against planktonic cells. For this reason, they needed AgNPs, which were synthesized during F. oxysporum (strain 511) reaction with 1 mM AgNO3 at 28 °C for 24 h. By this method, they obtained particles from 20 to 50 nm and proved that AgNPs have fungicidal effect against FLC-resistant C. albicans and are not cytotoxic to mammalian cells [65]. In 2017 Sonar et al [66] produced spherical AgNPs with 20–25 nm as mean size for inhibition of select waterborne human pathogens. 10 ml of fungal filtrates and 90 ml of 1 mM AgNO3 were reacted at room temperature to achieve total reduction [66].
Although the mechanism of synthesis of AgNPs by F. oxysporum has been presumably explained, this fungus can successfully produce other types of NPs of a determined size. In 2002 scientists developed a method of obtaining Cd NPs from 5 to 20 nm with F. oxysporum as biocatalyst and 10−3 M CdNO3 as the aqueous solution [36]. Reduction of  ions caused by fungal reductases indicated the formation of 8–40 nm AuNPs. The reaction was carried out in the dark in 10−3 M solution with 10 g of F. oxysporum [67]. 20 g of this fungus was also used for biosynthesis of SrCO3 NPs from 10 to 50 nm in 10−3 M SrCl2 solution and the source of carbonate ions was the microorganisms themselves [68].
ions caused by fungal reductases indicated the formation of 8–40 nm AuNPs. The reaction was carried out in the dark in 10−3 M solution with 10 g of F. oxysporum [67]. 20 g of this fungus was also used for biosynthesis of SrCO3 NPs from 10 to 50 nm in 10−3 M SrCl2 solution and the source of carbonate ions was the microorganisms themselves [68].
In 2004 researchers proved the extracellular method of zirconia NPs synthesis formed by secreted fungal proteins can hydrolyze aqueous  ions. ZrO2 NPs of a dimension of 3–11 nm were confirmed at that time [69]. In 2005, 2–5 nm silica NPs were produced extracellularly by F. oxysporum used in the bioleaching sand grains, crossed over a two-step process. The first stage included bioleaching of silica in the form of silicic acid by fungal proteins. The second stage constituted hydrolysis of silicate complex by other specific fungal proteins to nano-silica [3].
ions. ZrO2 NPs of a dimension of 3–11 nm were confirmed at that time [69]. In 2005, 2–5 nm silica NPs were produced extracellularly by F. oxysporum used in the bioleaching sand grains, crossed over a two-step process. The first stage included bioleaching of silica in the form of silicic acid by fungal proteins. The second stage constituted hydrolysis of silicate complex by other specific fungal proteins to nano-silica [3].
Platinum NPs ranging in size from 10 to 100 nm of hexagonal, circular or square shapes, were synthesized by intra- and extra-cellular processes in 2006. Best results were achieved at pH 9, 65 °C and 1 g biomass/10 ml solution [70]. Six years later, in 2012, researchers synthesized the same kind of NPs with use of the same fungi from H2PtCl6 solution, but of a size close to 15–30 nm and 20 g of biomass [5]. Zinc sulfide NPs of 42 nm were biosynthesized extracellularly from ZnSO4·H2O aqueous solution by F. oxysporum [71]. In 2017 Sandoval-Cárdenas et al obtained cadmium sulfide quantum dots of 2–6 nm diameter by incubation of F. oxysporum with a cadmium sulfate solution for 12 d at 30 °C [72]. It is astounding how one type of fungus can synthesize so many types of NPs, or contribute to the formation of one type, such as silver in various ways.
6. Measuring NPs
Depending on the sample, various methods for measuring NPs are applied. There are: optical and electronic methods, which include UV–vis and UV photoelectron spectroscopy; structural—scanning electron microscopy (SEM), transmission electron microscopy (TEM), scanning probe microscopy (SPM), x-ray diffraction (XRD); chemical—x-ray photoelectron microscopy (XPS), energy dispersive spectroscopy (EDX), temperature-programmed desorption; and vibrational—Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. By these methods, not only the size of the NPs be determined, but also their shape, surface construction, functional groups and many other features [73], as shown in table 2.
Table 2. Analyzers used to characterize the NPs with their applications and mechanisms. Reproduced from Karthik et al [73].
| Sample arrangement | Analyzer | Machinery | Utilization |
|---|---|---|---|
| Powder | FTIR | Obtaining light spectrum emitted by molecule | Detection functional groups synthesizing NPs |
| XRD | Retarded electrically charged particles of satisfactory energy | Phase arrangement crystal size and orientation in semicrystalline polymer | |
| XPS | Number of electrons fleeing from material exterior and kinetic energy | Counting elements surrounding sample surface, chemical and electronic state of elements | |
| Supernatant | Raman spectroscopy | Interacting with bonds' molecule electron cloud during the transition of light | Molecule fingerprint, crystal orientation |
| TEM | Sample crystallographic composition at an atomic scale | NPs orientation, size, exfoliation, distribution, intercalation and dispersion | |
| SPM | Exterior unevenness | NPs structure, properties and surfaces | |
| EDX | Combined with SEM | NPs quantity near and at the surface | |
| Temperature-programmed desorption | Heating and transferring exterior to the adsorbed species | Typifying oxide exteriors in view of acid sites | |
Many researchers have shown that atom transition of metal clusters indicates magnetic and electronic properties that differ significantly from those of bulk materials. Since most of the physical and chemical methods permit prediction of NPs size, they are used as precocious matter in towering technology. Furthermore, they can be used as sample matter for necessary tests that require nonaggregate, homogeneous particles with an expected size and confined size distribution [74]. Additionally, NPs because of their size, display differences in physical and chemical features, which in turn determine changes in optical features which can induce changes in color. For example, gold colloids emerge as dark red, with thermal adaptation, material hardiness, catalytic activity, conductivity and solubility [75].
6.1. Determining NPs size by dynamic light scattering
Dynamic light scattering (DLS) is one of the most widespread method used for measuring the size of NPs. The technique is also called photon correlation spectroscopy. During a DLS analysis, the suspension of metal NPs is followed by an electromagnetic wave, and as a consequence light encroaches on the NPs. A process called scattering modifies the orientation and intensity of the electromagnetic wave. Because of kinetic energy, metal NPs are in an unchanging accidental stir and their modification of intensity with time holds, therefore the data from accidental stir may be used to measure the diffusion of NPs coefficient. Determined by the NPs shape, for spherical particles, the hydrodynamic NP radius can be estimated by Stokes–Einstein mathematical statement [76]
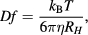
where  is diffusion coefficient,
is diffusion coefficient,  is Boltzmann constant,
is Boltzmann constant,  is temperature and
is temperature and  is viscosity of the surrounding media.
is viscosity of the surrounding media.
DLS works great in the case of monomodal samples, but for bimodal samples, researchers found that common DLS estimations do not precisely measure the NPs mixtures with a large distinction in the ratio between the diameter of NPs. The limit of size ratio outside of which bimodal size distinctions may not be thoroughly revealed has been reported in ratios of 2:1 to 3:1 [77].
DLS has gained approval for sizing metal NPs and has been used extensively to establish their hydrodynamic size. Time for DLS measuring is relatively short. Furthermore, the method is almost completely automated therefore, the entire procedure requires little work and experience. Also, as this technique is non-destructive, samples may be used for further measurements or testing [76].
Gold NPs are frequently used as signal amplification samples for converted DLS-based biosensors because of their higher light scattering ability. Researchers have been working on gold NP-based DLS immunoassay for ultrasensitive detection of Listeria monocytogenes in lettuces with success [78]. Balog et al have shown that it is possible to bypass restrictions in NPs characterization in optically biological or physiological matrices by using depolarized light scattering [79].
The size of silver NPs was successfully measured at ~35.4 nm by DLS when Trichoderma asperellum is used to synthesize them from pure silver nitrate. However, the size could be impaired due to inputs from hydrated capping agents and solvation effects [80]. Another possibility to obtain Ag NPs from the same solution is biosynthesis with A. flavus. It was confirmed by DLS that their size was 17 ± 5.9 nm [39]. Penicillium aurantiogriseum, P. citrinum and P. waksmanii were used to synthesize copper oxide NPs whose size ranged accordingly: 89–250 nm, 85–295 nm and 79–179 nm depending on the pH. The DLS diagram showed that copper NPs were created with well-documented dimensions and monodispersed [81].
6.2. Electron microscopy (SEM, TEM)
The SEM is a significant optical tool for the detection of bulk samples. An electron exploration is assembled by one-, two- or three-stage demagnification of the smallest cross-section of the electron unidirectional signal after hastening [82]. Atoms produce various signals while they interact with electrons, which produces information about the sample surface topography [83]. Analyzing tissue probes for accumulation of NPs by SEM correlating with optical microscopy is a splendid strategy to locate triple- modality magnetic resonance imaging, -photoacoustic imaging,—Raman imaging NPs, and determine their precise position in respect to biologically pertinent tumor tissue structures. In this case, SEM supplied high resolution analysis of tissue by the appearance of NPs [84].
F. oxysporum produced silica oxide NPs in a biotransformation process with rice husks as the biological raw material. Fungus was incubated at 28 °C at a pH of 6.8, which caused 80% of the silica to dissolve and 5000 nm particles to form. These analyses were confirmed by SEM combined with EDX [8]. A. parasiticus also demonstrates the ability to cause NPs biosynthesis. It was discovered that this fungus could efficiently synthesize silver NPs from a AgNO3 solution. SEM confirmed that AgNPs could create aggregates whose size greatly exceeds parameters obtained from TEM (5–60 nm) (El-Azis, 2014).
In TEM a sample is irradiated by an electron beam of equal prevailing density [82]. Because of TEMs high resolution, it is a supreme method for measuring NPs sizes. Notwithstanding, dilution process artifacts may not be eliminated, which is the reason of that only diluted samples can be investigated for imaging and characterization of engineered NPs in sunscreens [85].
Park et al demonstrated a hybrid method of reconstructing the NPs 3D structures by uniting three technological advancements from TEM [86]. In situ TEM tests of the lithiation of crystalline Si NPs and kinetics of this process at the single-particle level was shown by McDowell et al [87]. Rendition of low-dose methods from biological TEM, consolidated with the energy of the aberration-corrected transmission microscope, has disclosed the atomic structure of gold NPs, without any previous knowledge or supposition about their packing, a suitable model that should be widely used. This discovery opens the way for determining the other metal NPs structures and their organization [88].
Humicola sp. growing at 50 °C and pH 9 can successfully synthesize silver NPs from 1 mM AgNO3 solution. The NPs morphology was tested by TEM and showed very well dispersed spherical NPs, from 5 to 25 nm [42]. 6–18 nm gold NPs were synthesized by Nigrospora oryzae from HAuCl4 solution. This also showed the monodispersed spherical nature of biosynthesized Au NPs, which was confirmed by TEM and atomic force microscopy (AFM) [7].
These methods were selected as those most commonly used by scientists studying myco-nanosynthesis and by means of which they could get information about the size of the chosen NPs. There are more specialized methods, which are commonly used for determining NPs characteristics, however, they are not prevalent for fungal synthesis nowadays, but they could be successfully used there. Some of these are described below.
6.3. Flow field-flow fractionation (FFFF or AF4)
AF4 is an efficacious separation method that functions well for characterizing NPs in many fields, such as biological or environmental. Substantially, AF4's definition may be used to get information about NPs size from the calibration of conventional size negligent of the nature of particles. In fact, the interplay among NPs and the permeation membrane inside the separation channel may result in errors in the estimated particle measurements. In the same way, losses of matter in AF4 separation can usually make the measurements complicated and the improvement point is determined either on a size-by-size basis or by way of multidetector. AF4 is usually coupled with inductively coupled plasma mass spectrometry (ICP-MS), what can provide a combination of abilities for AF4 to separate NPs according to their hydrodynamic diameter with outstanding sensitivity and selectivity of ICP-MS detection [89]. Researchers determined that combine hollow-fiber FFFF with multi-angle light scattering is an exemplary hyphenated methodology for the contemporaneous size-separation and characterization of gold NPs samples. Some values defined by AF4 can be associated with median arcuate ligament syndrome (MALS) define values, which can produce information about NPs morphology [90]. Wagner et al had success with a common sample preparation scheme for inorganic engineered NPs in a complex matrix for apprehension, designation and quantification by asymmetric flow-field flow fractionation conjugated with multi-angle light scattering and ICP-MS [91]. Baalousha and Lead insinuate that only raw size data, e.g. mass, volume, number, intensity and average particle size can be obtained by AFM, AF4 and DLS and are equivalent for highly monodisperse hard spheres [92]. Barahona et al advised an approach using multimodal mixtures of monodispersed silica NPs standards with the use of the AF4-ICP-MS method for the coincident size and concentration ascertainment of this kind of NPs in an aqueous suspension [89].
6.4. ICP-MS
ICP-MS is a conventional method for total quantification of the cellular enlistment of metal or metal oxide and offers high sensitivity and selectivity for elemental analysis. Before analysis, it is important to perform cellular membrane disintegration. Peak intensity depends on the size of NPs [93]. Single particle ICP-MS was investigated for detection and characterization of NPs and as a result evaluation instrument. Single part ICP-MS coupled with a single particle calculation tool for result processing is a simple and quick screening method able to detect and characterize metal and metal oxide NPs at low concentration conditions. In this case, researchers worked with four types of NPs and two types of ICP-MS, where size detection limits were 20 nm for gold and silver, 50 nm for titanium oxide and 200 nm for silica for a quadrupole instrument [94]. Another research paper shows how to develop and validate single particle ICP-MS for sizing and quantitating silver NPs in chicken meat [95].
7. Conclusion
Fungal synthesis of NPs is the cheapest, most environmentally friendly method, which does not require a huge expenditure of energy in comparison to other methods. The ability to synthesize NPs of determined size using fungi is recognized as a tremendous step forward. Researchers have optimized conditions in many ways to be able to manipulate this feature. The most commonly used methods for the detection of NPs size are TEM and SEM analyzers, because they are not complicated to use and results are easy to analyze. The most important area where NPs size is significant, is for determining their application, for when we know the size of an element or compound, we can precisely define its future uses. F. oxysporum dominates NPs production because of its wide range of applications, cost effectiveness, simplicity of use and management, and superior qualities. Its potential use as an effective solution for industrial production of NPs should be more widely publicized.
Acknowledgments
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wroclaw University of Science and Technology.