Abstract
Polycyclic aromatic hydrocarbons (PAHs) in water are classified as organic micropollutants, which are carcinogenic even in very low concentration (ppb). In this study the green synthesized iron oxide nanoparticles (IONPs) were green synthesized at room temperature by using pomegranate peel extract. The green synthesized IONPs were used for adsorbing benzo(a)pyrene and pyrene (PAHs) from water. Factors affecting the adsorption were investigated. These factors are: nanoparticles dose, pH, temperature, and initial concentration of PAHs. The overall results showed that the maximum adsorption capacities of IONPs towards pyrene and benzo(a)pyrene were 2.8 and 0.029 mg g−1, respectively. The thermodynamic study indicated an exothermic adsorption process of pyrene and benzo(a)pyrene. The kinetic and isotherm studies were carried out. The obtained data revealed that the adsorption process follows a pseudo-second order mechanism and obeys Langmuir isotherm model. In addition, the IONPs proved to be a potential candidate for the adsorption of pyrene and benzo(a)pyrene even after five cycles of use and regeneration. The investigation was extended using semi-pilot plant to remove the studied PAHs from artificially contaminated water. The results showed that the IONPs was capable to remove the pyrene and benzo (a) pyrene at the rate of 98.5 and 99%, respectively. It also can be used as disinfectant.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene and other are known for being widespread environmental contaminants. They are formed during incomplete combustion or pyrolysis of organic material. Generally, these substances are well detected and are found in air, water, soils and sediments, at very low concentrations except near their point sources. PAHs are present also in some foods and in certain pharmaceutical products as micropollutants. In addition, tobacco smoke contains high concentrations of PAHs [1]. It was reported by the Environmental Protection Agency's (EPA's) Guidelines for Carcinogen Risk Assessment [2] that benzo(a)pyrene is carcinogenic to humans. This conclusion is based on the strong and consistent evidence of an extensive number of studies demonstrating the carcinogenicity in multiple animal species that exposed to these micropollutants via all routes of administration. Meanwhile, the exposure of man to different PAHs mixtures containing benzo(a)pyrene increases the cancer risks in the lung and skin. These evidences include formation of specific DNA adducts and characteristic mutations in oncogenes and tumor suppressor genes that have been observed in humans exposed to PAHs mixtures. This combination of human, animal, and mechanistic evidence provides the basis for characterizing benzo(a)pyrene as carcinogenic to humans. It is well known that benzo(a)pyrene (BaP) is the greatest carcinogen PAHs [3].
The effluent of PAHs from the petrochemical industry wastewater, petroleum refinery wastewater, and continuous leakage of the fuel from the underground old and cracked gasoline storage tanks in urban areas, are the main sources of PAHs contamination of surface and groundwater [4, 5]. The Federal Environmental Agency of Germany [6] studied the inputs of PAHs to the environment. Emissions into the atmosphere are of the greatest significance input of PAHs. More than 80% of the PAHs input to the environment are thus influenced by atmospheric deposition. The direct deposits on surface water is an additional input to the environment. Substances first deposited onto the urban ground are washed out into waterways via erosion and surface runoff [3, 6]. According to the adopted standards by the European Union (EU), the maximum allowable concentration of PAHs in drinking water is 200 ng l−1 [7]. In 2005, EPA has established ambient water quality criteria to protect human health from the carcinogenic effects of PAHs exposure. The goal of these criteria was to set a non-detectable level (zero concentration of carcinogenic PAHs in surface water).
In Egypt, the total PAHs concentration in fresh Nile water at the Rosetta branch is greatly varied from 242 to 732 ng l−1 with the mean value of 408 ng l−1. The concentration in the wastewater ranged from 894 to 1979 ng l−1, and the mean value is 1476 ng l−1 [8]. The level of 13 PAHs in water samples collected from 6 different locations through the governorate of El-Menofiya were found to range from 226.9 ng l−1 at El Sarsawia canal to 1492.2 ng l−1 at El Menofi drain. These PAHs were found to be predominated by three- and four-ring PAHs [9]. In addition, the residual fractions of different 16 PAHs in surface water near the coast of Alexandria, Egypt were investigated during 2013–2014. The determined average PAHs values of these 16 PAHs ranged from 1223 to 11183 ng l−1, the average value is 4326 ng l−1 [10]. The average concentrations of the 16 PAHs ranged from 1270 to 17300 ng l−1 at three different sites in the River Nile and Esmailia canal during summer and winter seasons [11].
On the other hand, nanotechnology has become an important field recently in the modern science. The nanoparticles characterized by structure ranging from 1 to 100 nm in size. The nanotechnology deals with the synthesis, manipulation and strategy of these nanoparticles [12–15]. In the modern research, nanotechnology is swiftly gaining renovation in varied fields, including biomedical, health care, cosmetics, drug-gene delivery, food and feed, health, mechanics, environmental, energy science, optics, light emitters, nonlinear optical devices, photo electrochemical applications single electron transistors, electronics, catalysis, chemical industries and space industries [16]. Green synthesis, in addition, is a bottom-up approach in which the atoms and molecules assemble together in the form of nanosized structure. It is a single step process where no reducing agent or polymers is added. Natural sources of polyphenols are also important in the green synthesis process. The advantages of the green synthesis over the physical or chemical synthesis are: cost effective and environmentally friendly, and easy to scale up the scientist nanoparticles. In addition, there is no need to use energy, pressure and high temperature. Furthermore, different material from natural bio-renewable sources can be used for synthesis [13, 16, 17].
On the other hand, iron oxide nanoparticles (IONPs) are naturally occurring in the environment in the rock deposits, in the soil and mountains. They are chemical compounds of iron and oxygen. The magnetite (Fe3O4) and the maghemite (γ-Fe2O3) particles are the most common pure iron oxide. Due to their nontoxicity and magnetic properties, the IONPs are considered safe nanoparticles and one of the most multipurpose nanomaterials that are used in medical applications [18]. The existence of polyphenols in the green synthesis of IONPs is required. Such polyphenols can be found in vitamins, coffee, tea, wine and proteins [19, 20]. Furthermore, IONPs can be obtained from different plant extracts including Eucalyptus globulus leaf [21], banana peel ash [22], and pomegranate leaf [23].
The aim of the present investigation is to study the removal of benzo(a)pyrene and pyrene (as PAHs) from water via the adsorption. Green synthesis of IONPs adsorbent using pomegranate peel extract as inexpensive, and ecofriendly method was suggested. A further aim is to investigate the thermodynamic, kinetic and isotherm parameter of the adsorption process. The batch experiments were conducted to investigate the factors affecting the adsorption of benzo(a)pyrene and pyrene. Different nanoparticle doses, temperature, pH, the initial concentration of benzo(a)pyrene and pyrene, and the effect of different contact times have been examined. The green synthesized IONPs was characterized using x-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscope (HRTEM), Fourier transform infrared (FTIR) spectrometry and energy dispersive x-ray (EDX) spectroscopy. A semi pilot-plant was used for the removal of benzo(a)pyrene and pyrene from artificially contaminated tap water in a batch flow system. Thus the obtained information can be useful for the removal of PAHs from water in case of the presence of such pollutants. The concentration of benzo(a)pyrene and pyrene was detected and followed using high performance liquid chromatography (HPLC) during the entire experimental investigation.
2. Experimental
2.1. Chemicals
Ferrous sulfate, sodium hydroxide, methanol, pyrene, benzo(a)pyrene and acetone, were purchased from Sigma-Aldrich, Germany. The stock solutions of pyrene and benzo(a)pyrene were prepared in acetone. Series of variable concentrations were prepared by the appropriate dilutions with distlled water.
2.2. Preparation of pomegranate peel solid waste extract
An amount of 2.0 g of the dried pomegranate peel solid waste was placed in 200 ml beaker, followed by the addition of 100 ml of deionized water. The mixture was gently heated until the color of the aqueous solution changes to dark yellow. The extract was cooled to room temperature and filtered through a Whatman filter paper (no. 40). Phytochemical screening of pomegranate peel extract was qualitatively estimated according to the method described by Selvaraj et al [24].
2.3. Green synthesis of iron oxide nanoparticles using pomegranate peel extract
An amount of 4.0 g ferrous sulfate was added to 1 l of distilled water. The mixture was stirred vigorously for 10 min, till complete dissolution of ferrous sulfate. A 20 ml aliquot of the previously prepared pomegranate peel solid waste extract was added to the ferrous sulfate solution. The mixture was stirred in a very low speed (20 rpm). A solution of 2.0 M NaOH was drop wise, added until the pH increased to 12. The mixture was further continuously stirred at low speed (20 rpm) to allow the precipitation of IONPs. The obtained dark brown precipitate was collected and washed several times with double distilled water. The centrifuge was utilized after each step of washing in order to collect the synthesized IONPs. The collected nanoparticles were left to dry overnight at 70 °C to obtain the IONPs powder. To confirm the role of the pomegranate peel extract on the green synthesis of IONPs, the above preparation procedure was repeated without the addition of this extract. The obtained precipitate was collected and dried at 70 °C overnight. The prepared precipitate was then examined.
2.4. Characterization of iron oxide nanoparticles
The crystallinity of the synthesized IONPs was investigated by XRD (PANalytical–Empyrean) under the following conditions: scan axis: Gonio, start position 2θ = 5.0129°, end position 2θ = 79.9709°, step size 2θ = 0.0260°, scan step time is 18.8700 sec, scan type: continuous, PSD length 2θ = 3.35°, measurement temperature is 25 °C, anode material: Cu-Kα1 is 1.540 60 Å, Cu-Kα2 is 1.54443 Å, Cu-Kβ is 1.392 25 Å, ratio Kα2/Kα1 = 0.500 00, generator settings: 30 mA, 45 kV. SEM (quanta FEG 250) was employed to examine the surface morphology of the synthesized IONPs. In addition, EDX (AMETEK elemental analysis device) was used for the characterization of IONPs. Furthermore, HRTEM (JEOL JEM–2100 electron microscope) was used to gain the actual particle size of the synthesized IONPs.
For the detection of benzo(a)pyrene and pyrene concentrations, the high performance liquid chromatograph (HPLC) was used (Agilent 1200 HPLC). It is equipped with a 5 µm × 25.0 cm × 4.6 mm LC-C18 column, PDA detector and auto-sampler. The mobile phase consisted of 40% water and 60% acetonitrile. The solvent program was isocratic, the flow rate was 1.0 ml min−1 and the injection volume was 5 µl.
2.5. Batch adsorption of pyrene and benzo(a)pyrene by the green synthesized IONPs
2.5.1. Determination of IONPs optimum adsorption dose.
Distilled water was artificially by contaminated with a combination of benzo(a)pyrene and pyrene at concentration of 1 µg l−1 and 100 µg l−1, respectively. A jar-test apparatus was used in a batch experiment in this study. The experiments were carried out at room temperature and pH 7. The effect of different doses of the green synthesized IONPs namely: (10, 30, 50, 70, 90, 110 and 130 mg l−1) was investigated. The experiment was carried out under a fast mixing (300 rpm) for 5 min, followed by slow mixing at 150 rpm. The contact of iron oxide and the contaminated water was for 30 min. The jar-test was stopped, and the solution was centrifuged in order to collect the nanoparticles. The concentration of pyrene and benzo(a)pyrene in the supernatant was detected by HPLC.
2.5.2. Study the effect of pH, temperature, contact time, TDS and initial concentration of PAHs on the efficiency of adsorption.
The series of experiments were conducted using artificially contaminated distilled water with 100 µg l−1 pyrene and 1 µg l−1 benzo(a)pyrene, with the optimal dose IONPs for 30 min contact time. The following variables were studied:
- (a)Effect of pH: this study was carried out at different pH ranging from 4 to 8 and at room temperature (22 °C).
- (b)Effect of temperature: this study was carried at different temperature ranging from 20 to 50 °C, using a controlled temperature water bath, and at pH 7.
- (c)Effect of contact time: variable contact time ranging from (30, 60, 90, 120, 150, 180, 210 and 240 min) were studied at room temperature and pH 7.
- (d)The effect of total dissolved solids (TDS): in this investigation, NaCl, in the range of 0–3.5% was added to prepare solutions of the required TDS. The experiment carried out with a solution containing 300 µg l−1 pyrene and 3 µg l−1 benzo(a)pyrene at150 min contact time, different room temperature and at pH 7.
- (e)The effect of the initial PAHs concentrations: different concentrations of benzo(a)pyrene (1, 1.5, 2, 3, and 4 µg l−1) of pyrene (100, 150, 200, 250, 300, 350 and 400 µg l−1) were used. The study was conducted at 150 min contact time, room temperature and pH 7.
2.6. Regeneration of iron oxide nanoparticles
In order to perform the adsorption process more economically, it was important to study the reuse of the adsorbent. The regeneration and reuse of the green synthesized IONPs was investigated as follows: a mixed solution of 3 µg l−1 of benzo(a)pyrene and 300 µg l−1 pyrene was allowed to be in contact with 90 mg l−1 green synthesized IONPs at pH 7 and room temperature for 150 min. The green synthesized IONPs were then separated by centrifugation. The collected green synthesized IONPs were rinsed 3 times with 3 ml methanol each to remove pyrene and benzo (a) pyrene. The concentrations of pyrene and benzo(a)pyrene in the separated supernatant solutions were determined by HPLC, until no pyrene and benzo(a)pyrene were detected. The rest of the methanol supernatant solution that contains pyrene and benzo(a)pyrene was submitted to rotary evaporator to get rid of the methanol. The obtaind solid pyrene and benzo(a)pyrene could be reused again.
The regenerated IONPs were reused again for adsorbing of pyrene and benzo(a)pyrene for several times according to the previously described procedure. The purpose is to investigate the effect of IONP reuse on the adsorption capacity. The regeneration and reuse process was repeated 5 times.
2.7. Semi-pilot plant for the removal of benzo(a)pyrene and pyrene from water
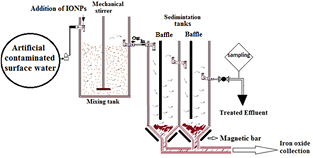
To study the efficiency of the iron oxide nanoparticles for the removal of the studied contaminants from drinking water, a semi-pilot plant was designed for a batch flow system (figure 1). Drinking water samples were brought to the pilot plant, where water was artificially contaminated with benzo(a)pyrene and pyrene at the concentration of 1 µg l−1 of benzo(a)pyrene and 100 µg l−1 pyrene. The physical and chemical characteristics of this water were carried out according to the standard methods [25].
Figure 1. The continuous treatment system for the removal of PAHs from artificial contaminated surface water.
Download figure:
Standard image High-resolution imageThe semi-pilot plant (figure 1) consists of a mixing tank (1.0 × 0.8 × 0.8 m3) and is equipped with mechanical stirrer in middle of the tank. In this mixing tank the artificial contaminated tap water was placed and the iron oxide nanoparticles were added at the pre-determined dose, namely, 110 mg l−1. The stirrer is to serve for the complete mixing of the contaminated tap water with the iron oxide nanoparticles. The mixture was stirred for 150 min, continuously, after which the stirred mixture was allowed to flow into the first sedimentation tank followed by the second sedimentation tank. Each sedimentation tank is partitioned by baffle to allow the settling down of the particles. The dimensions of each sedimentation tank are (0.8 × 0.35 × 0.35 m3). Each sedimentation tank is equipped with two magnetic bars in the bottom to enhance the settling of the iron oxide nanoparticles to the bottom of the tanks. The final treated water was then collected for physical and chemical analyses. The settled iron oxide nanoparticles were collected and submitted for regeneration and reuse as described above.
3. Result and discussion
By applying the qualitatively estimated methods [24], the phytochemical constituents of pomegranate peel aqueous extract were found to consist of tannins, saponins, quinones, terpenoids, steroids, flavonoids, phenols, alkaloids, cardiac glycosides, coumarins and betacyanin. The glycosides and anthocyanin were not found in the pomegranate peel aqueous extract.
3.1. Characterization of iron oxide nanoparticles
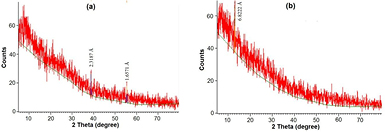
Figure 2 illustrates the XRD pattern of the green syntheize iron oxide nanoparticles. There are two small observed peaks at 2θ positions, namely: 38.8062° and 55.4002° refers to Fe2O3 (figure 2(a)). This observation is an indication of the amorphous structure of the green syntheized IONPs. It has been reported that amorphous metal oxides show great industrial potential in solar energy transformation, sorption, purification processes and catalysis. In such applications, amorphous iron (III) oxide plays a key role. This is mainly due to its superior catalytic activity, super-paramagnetic behavior, and the larger specific surface area of the nano-particles. The super-catalytic behavior of the amorphous IONPs is more effective than the nano-crystalline, polymorphs or particles of metallic iron of the same diameter [26]. On the other hand, figure 2(b) shows the XRD pattern of the chemically synthesized iron oxide particles. It is only one small peak at 2θ = 12.9662°, as an indication of the amorphous formation of iron hydroxide particles.
Figure 2. XRD pattern of (a) the green synthesized iron oxide nanoparticles, and (b) chemically synthesized iron hydroxide particles.
Download figure:
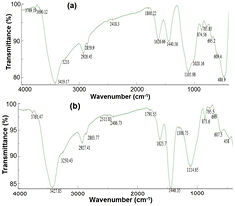
Standard image High-resolution imageMeanwhile, figure 3 illustrates the FTIR spectra of green synthesized particles and chemically synthesized particles, respectively. In this figure, the presence of phytochemical compounds on the surface of the green synthesized particles, indicates the following differences:
- (1)Appearance of a new low intensity peak at 3690.12 cm−1 (due to free OH stretching vibration, mainly for phenols), 3233 cm−1 (due to intermolecular hydrogen bond stretching, vibration), 2418.3 cm−1 (due to C–H stretching, vibration), 1800.22 cm−1 (due to C=O stretching, vibration), high intensity peak at 1105.98 cm−1 (due to C–N stretching, vibration), and small peak at 1020.16 cm−1 (due to C–O H stretching vibration), 785.85 cm−1 (due to =C–H=CH2, out-of-plane, bending vibration).
- (2)The shift of the peak at 3761.47 cm−1 (due to vibrational overtones from charge transfer between O and Fe3+) to 3769 cm−1, and high intensity peak at 1446.35 cm−1 (due to Fe–OH bending vibration) to low intensity peak at 1440.56 cm−1.
- (3)Disappearance of the high intensity peak at 3427.85 cm−1 (due to OH (involved in hydrogen bond) stretching vibration), low intensity peak 3250.43 cm−1 (due to stretching vibration of OH involved in hydrogen bond), 2511.83 cm−1 (due to O–H stretching vibration), 2406.73 cm−1 (due to O–H stretching vibration), 1791.55 cm−1 (due to OH bending vibrations), the low intensity peak at 1300 cm−1 (due to O–H, in-plane, bending) vibration), the high intensity peak at 1114.65 cm−1 (due to OH–groups, in-plane deformation, bending vibration), 795.5 cm−1 (due to OH bending vibration) and the peak at 458 cm−1 (due to lattice vibrations involving OH−).
- (4)High intensity peak at 486.9 cm−1 refers to Fe–O stretching vibration.
Figure 3. FTIR spectra of (a) green synthesized iron oxide nanoparticles, and (b) chemically synthesized iron hydroxide particles.
Download figure:
Standard image High-resolution imageFurthermore, the SEM images (figures 4(a) and (b)) show the surface morphology of green synthesized particles and chemically synthesized particles, respectively. Figure 4 indicates a difference in the surface morphology. The surface of the green synthesized particles (figure 4(a)) is more porous than the chemically synthesized iron hydroxide particles (figure 4(b)).
Figure 4. SEM images at magnification 16 000 of (a) green synthesized iron oxide nanoparticles, and (b) chemically synthesized iron hydroxide particles.
Download figure:
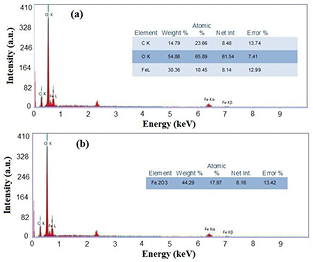
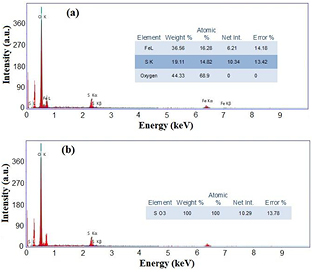
Standard image High-resolution imageIn addition, EDX spectrum (figure 5(a)) gives the elemental analysis of the green synthesized iron oxide in the metallic form. The atomic weight percentages of iron and oxygen were 30.36% and 54.86%, respectively, with a small percentage of carbon (14.79%). The obtained results confirm the presence of carbon on the surface of the green synthesized iron oxide, that comes from the phytochemicals of the pomegranate extract. Figure 5(b) also indicates that the EDXs of the green synthesized iron oxide is in the oxide form. Here, the oxidized form is Fe2O3 at weight percentage of 44.29%. Figure 6(a), at the mean time, represents the EDXs of the chemically synthesized particles under the same conditions. Furthermore, the atomic weight percentages were 36.56% iron, 44.33% oxygen with a small percentage of sulfur (19.11%). Meanwhile, the oxide form in figure 6(b) shows that the iron oxide was not formed, besides, sulfur was in the oxide form as SO3. These facts reveal that the obtained particles were not iron oxide, but it is iron hydroxide particles as indicated from XRD.
Figure 5. EDXs of green synthesized iorn oxide in (a) metal form and (b) oxide form.
Download figure:
Standard image High-resolution imageFigure 6. EDXs of chemiclly synthesized iorn hydoxide in (a) metal form, and (b) oxide form.
Download figure:
Standard image High-resolution imageFurther investigation was conducted for the determination of the actual particle size of the green synthesized IONPs using HRTEM (figure 7(a)). The average particle size was about 2.7 nm. This image (figure 7(a)) shows that the green synthesized IONPs has a narrow size distribution. This reveals that such particles have disordered arrangement, and the green synthesized IONPs are amorphous. These results are compatible with the XRD results. Small nanosize particles strongly favored formation of agglomerates due to the high density number of these paricles, while larger ones stay mainly unagglomerated [27]. According to Weatherill et al [28] the iron oxide nanoparticles with small nano size have the tendency to form agglomerates. This agglomeration is caused by the magnetostatic (magnetic dipole–dipole) interactions between the iron oxide nanoparticles [29]. Using a higher magnification (figure 7(b)), it is possible to detect the lattice planes of individual particles, where the interplanar spacing is 0.35 nm.
Figure 7. (a) HRTEM image, and (b) visible lattice planes of the green synthesized iron oxide nanoparticles.
Download figure:
Standard image High-resolution image3.2. Batch adsorption of pyrene and benzo(a)pyrene using green synthesized IONPs
At equilibrium qe (mg g−1), the maximum amount of PAHs adsorped on the surface of the green synthesized IONPs was calculated according to the following equation
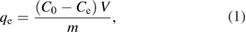
where C0 (mg l−1) is the initial concentration of pyrene or benzo(a)pyrene, and Ce (mg l−1) is the concentrations of pyrene or benzo(a)pyrene at equilibrium. V(l) is the volume of the solution containing pyrene and benzo(a)pyrene (in combination), and m(g) is the mass of the green synthesized IONPs.
The percentage of pyrene or benzo(a)pyrene removal (R) was calculated as follows
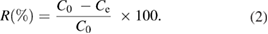
3.3. Factors affecting the adsorption of pyrene and benzo(a)pyrene
3.3.1. Effect of iron oxide dose on the adsorption process.
The effect of different doses of the green synthesized IONPs on the adsorption of pyrene and benzo(a)pyrene was investigated. The examined doses of the IONPs ranged from 10 to 110 mg l−1. The obtained results are given in table 1. These results reveal that the percentage of removal of pyrene and benzo(a)pyrene increased by increasing the dose of IONPs from 10 to 90 mg l−1. A steady state of removal was reached upon using 90 mg l−1. Thus, 90 mg l−1 was considered the optimum dose of the IONPs.
Table 1. Effect of iron oxide nanoparticles doses on the adsorption process of pyrene and benzo(a)pyrene at contact time 30 min, pyrene concentration C of pyrene is 100 µg l−1, of benzo(a)pyrene is 1 µg l−1, room temperture and pH 7.
| IONPs dose (mg l−1) | Parameter | |||
|---|---|---|---|---|
| Pyrene | Benzo(a)pyrene | |||
| C (µg l−1) | R (%) | C (µg l−1) | R (%) | |
| 0 | 100 | — | 1 | — |
| 10 | 70 | 30 | 0.8 | 20 |
| 30 | 45 | 55 | 0.52 | 48 |
| 50 | 39.4 | 60.6 | 0.34 | 56 |
| 70 | 36.4 | 63.6 | 0.35 | 65 |
| 90 | 34 | 66 | 0.3 | 70 |
| 110 | 33 | 67 | 0.29 | 71 |
| 130 | 32.2 | 67.8 | 0.28 | 71.6 |
3.3.2. Effect of pH on the adsorption process.
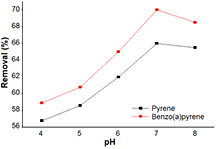
The pH of the tested solution plays an important role in water chemistry. It affects the properties of the solutes as well as the surface charge of the adsorbents. Figure 8 shows the effect of different pH (from 4 to 8) on the removal efficiency of pyrene and benzo(a)pyrene. At these pH levels, no change was observed in the solution, but above pH 8, the color of the solution changed into light brown indicating the dissociation of IONPs. The results (figure 8) indicate that the percentage of removal decreased as the pH increased. The optimum pH for the adsorption is 7 (i.e. the neutral pH is the optimum pH for the adsorption). This could be attributed to the variable solubility of pyrene and benzo(a)pyrene at different pH values [30].
Figure 8. Effect of pH on the removal of pyrene (100 µg l−1) and benzo(a)pyrene (1 µg l−1), at room temperture, 30 min contact time and iron oxide nanopaticles dose of 90 mg l−1.
Download figure:
Standard image High-resolution image3.3.3. Effect of temperature on the adsorption process.
Figure 9 shows the effect of different temperatures (from 20 to 50 °C) on the adsorption of pyrene and benzo(a)pyrene. The results indicate that the adsorption decreased by increasing the temperature (figure 9). The optimum temperature was 20 °C (i.e. the adsorption can efficiently occur at room temperature).
Figure 9. Effect of temperature on the adsorption process of pyrene and benzo(a)pyrene at iron oxide nanopaticles dose of 90 mg l−1, contact time 30 min and pH 7.
Download figure:
Standard image High-resolution image3.3.4. Effect of contact time on the adsorption process.
The effect of variable contact times (from 30 to 240 min) on the adsorption of pyrene and benzo(a)pyrene using the green synthesized IONPs was investigated. The results are summarized in table 2 which show that the adsorption of pyrene and benzo(a)pyrene on the surface of IONPs increased by increasing the contact time from 30 min to 150 min. A further increase in the contact time over 150 min exhibited a slight enhancement in the adsorption. Thus, the optimum contact time was considered to be 150 min.
Table 2. Effect of contact time on the adsorption of pyrene and benzo(a)pyrene on the surface of green synthesized iron oxide at iron oxide nanoparticles dose 90 mg l−1, concentrations of pyrene is 100 µg l−1 and of benzo(a)pyrene is 1 µg l−1, room temperture and pH 7.
| Contact time (min) | Parameter | |||
|---|---|---|---|---|
| Pyrene | Benzo(a)pyrene | |||
| C (µg l−1) | R (%) | C (µg l−1) | R (%) | |
| 30 | 34 | 66 | 0.3 | 70 |
| 60 | 14.3 | 85.7 | 0.23 | 85 |
| 90 | 8.5 | 91.5 | 0.17 | 90 |
| 120 | 5 | 95 | 0.1 | 94 |
| 150 | 2 | 98 | 0.03 | 97 |
| 180 | 1.9 | 98.1 | 0.02 | 98 |
| 210 | 1.7 | 98.3 | 0.015 | 98.5 |
| 240 | 1.5 | 98.5 | 0.01 | 99 |
3.3.5. Effect of initial concentration of pyrene and benzo(a)pyrene on the adsorption process.
Initial concentrations of the of pyrene and benzo(a)pyrene were prepared in the range of 100–400 µg l−1 and 1–4 µg l−1, respectively. This range was selected according to the maximum solubility and detection limits of the studied PAH compounds. The effects of the initial concentration of pyrene and benzo(a)pyrene on the adsorption of each one; separately; was studied. The results are given in table 3. The obtained data indicated that increasing the concentration of pyrene and benzo(a)pyrene, increases the adsorption capacity of the studied PAHs micro-pollutants. Increasing the concentration of pyrene and benzo(a)pyrene leads to the presence of more π–π interaction, which indicates that the equilibrium reaction leads to the efficient removal [31].
Table 3. Effect of intial concentration of pyrene and benzo (a) pyrene on the adsorption process at iron oxide nanoparticles dose 90 mg l−1, contact time 150 min, room temperture and pH 7.
| Initial concentration of pyrene (µg l−1) | C (µg l−1) | R (%) | Initial concentration of benzo(a)pyrene (µg l−1) | C (µg l−1) | R (%) |
|---|---|---|---|---|---|
| 100 | 2 | 98 | 1 | 0.01 | 99 |
| 150 | 15 | 90 | 1.5 | 0.07 | 95.3 |
| 200 | 27 | 86.5 | 2.0 | 0.15 | 92.3 |
| 250 | 37 | 85.2 | 2.5 | 0.24 | 90.4 |
| 300 | 48 | 83 | 3.0 | 0.35 | 88.3 |
| 350 | 63 | 82 | 3.5 | 0.54 | 84.6 |
| 400 | 87 | 78.3 | 4.0 | 0.82 | 79.5 |
3.3.6. Effect of total dissolved solids on the adsorption process.
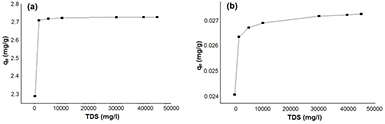
Total dissolved solids (TDS) plays an important role in water quality [32]. Thus, the effect of the total dissolved solids on the adsorption capacity was investigated under the optimized conditions. Figure 10 illustrates that increasing the level of NaCl increases the adsorption capacities of pyrene and benzo(a)pyrene. It was previously reported that the presence of inorganic ions (including Ca2+, K+, Na+,  and Cl−) binds water molecules tightly into hydration shells; thus; reduces the solubility of PAHs in water [33]. Therefore, the cavity volume that accommodates organic solute, forces the PAHs molecules onto the surface of the adsorbent [33]. This is mainly due to the fact that the presence of the inorganic salts increases the ionic strength of the aqueous solution, and affects the solubility of the organic molecules. This is known as salting-out effect (i.e. the engagement of water molecules around the ionic salt that reduces the available water and affect the dissolution of the PAHs [33].
and Cl−) binds water molecules tightly into hydration shells; thus; reduces the solubility of PAHs in water [33]. Therefore, the cavity volume that accommodates organic solute, forces the PAHs molecules onto the surface of the adsorbent [33]. This is mainly due to the fact that the presence of the inorganic salts increases the ionic strength of the aqueous solution, and affects the solubility of the organic molecules. This is known as salting-out effect (i.e. the engagement of water molecules around the ionic salt that reduces the available water and affect the dissolution of the PAHs [33].
Figure 10. Effect of total dissolved solids (TDS) on the adsorption process of (a) pyrene (300 µg l−1) and (b) benzo(a)pyrene (3 µg l−1) on the surface of the green synthesized iron oxide nanoparticles, at iron oxide nanoparticles dose 90 mg l−1, contact time 150 min, room temperture and pH 7.
Download figure:
Standard image High-resolution image3.4. Adsorption thermodynamic investigation
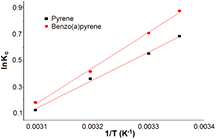
The values of Gibbs free energy ΔG0 (kJ mol−1), enthalpy ΔH0 (kJ mol−1) and entropy change ΔS0 (K J mol−1), of the adsorption process of pyrene and benzo(a)pyrene on the surface of IONPs, were evaluated according to the following equations

where Cads is the concentration (mg l−1) of pyrene and benzo(a)pyrene adsorbed on the surface of the IONPs at equilibrium, and Ce is the concentration (mg l−1) of pyrene and benzo(a)pyrene remained in the solution at equilibrium.
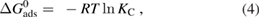
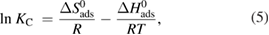
where  (K J mol−1) is the standard enthalpy, and
(K J mol−1) is the standard enthalpy, and  (J mol−1 K−1) is the entropy of the adsorption process. These values were evaluated according to equation (5). By plotting
(J mol−1 K−1) is the entropy of the adsorption process. These values were evaluated according to equation (5). By plotting  versus
versus  (figure 11), a straight line was obtained where
(figure 11), a straight line was obtained where  is the slope, R = 8.314 J mol −1 K−1 is the gas constant and T is the temperature (K).
is the slope, R = 8.314 J mol −1 K−1 is the gas constant and T is the temperature (K).
Figure 11. Thermodynamic plot of the adsorption process of pyrene and benzo(a)pyrene by using iron oxide nanoparticles.
Download figure:
Standard image High-resolution imageThe thermodynamic parameters are given in table 4. The negative value of  indicates the spontaneous nature of the adsorption process. The negative value of
indicates the spontaneous nature of the adsorption process. The negative value of  indicates the exothermic nature of the adsorption process. The negative value of
indicates the exothermic nature of the adsorption process. The negative value of  indicates the change in the randomness at the IONPs interface during the adsorption process.
indicates the change in the randomness at the IONPs interface during the adsorption process.
Table 4. Thermodynamic parameters of the adsorption process of pyrene and benzo(a)pyrene using of green synthesized iron oxide nanoparticles.
| Temperature (°C) | Thermodynamic parameters | |||||
|---|---|---|---|---|---|---|
| Pyrene | Benzo(a)pyrene | |||||
| −ΔG (J mol−1) | ΔH (J mol−1) | ΔS (J mol−1 K−1) | −ΔG (J mol−1) | ΔH (J mol−1) | ΔS (J mol−1 K−1) | |
| 20 | −1.7 | −17.2 | −52.68 | −2.17 | −22.13 | −67.07 |
| 30 | −1.4 | −1.78 | ||||
| 40 | −0.95 | −1.10 | ||||
| 50 | −0.34 | −0.50 | ||||
3.5. Kinetic investigation
The kinetic study on the adsorption of pyrene and benzo(a)pyrene on the surface of green synthesized IONPs was investigated according to the Langmuir–Hinshelwood kinetic model [34]. Langmuir pseudo first order (equation (6)), and pseudo second order kinetic models (equation (7)) were applied to obtain the adsorption rate constants.

where qe (mg g−1) and qt (mg g−1) are the amount of pyrene and benzo(a)pyrene adsorbed on the surface of IONPs at equilibrium and t(min), respectively. K1 (min−1) is the rate constant in the pseudo-first-order adsorption process. By plotting 1/qe against 1/t the value of K1 and qe can be determined
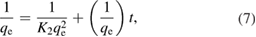
where K2 (g mg−1 min) is the pseudo-second-order rate constant. By plotting 1/qe against t, the K2 and qe can be determined. The initial h0 (mg g−1 min) is defined as follows:

Figures 12(a) and (b) exhibit the pseudo-first-order plot of pyrene and benzo(a)pyrene, respectively. Meanwhile, figures 12(c) and (d) give the pseudo-second-order plot of pyrene and benzo(a)pyrene, respectively. The calculated values, of the rate constants (K1, K2), h0, qe, and the correlation coefficient of both models, are given in table 5. The pseudo-second-order model plot is linear, based on the assumption that the rate-limiting factors is chemisorption. According to Robati [35] the removal from a solution is due to physico-chemical interactions between the two phases.
Table 5. Rate constants of thepseudo-first-order and pseudo-second-order kinetic models for the adsorbtion of pyrene and benzo(a)pyrene.
| Kinetic model | Pyrene | Benzo(a)pyrene |
|---|---|---|
| Pseudo-first-order kinetic | ||
| R2 | 0.98 | 0.887 |
| K1 (min−1) | 18.9 | 15.7 |
| qe (calculation) (mg g−1) | 0.992 | 0.0094 |
| qe (experiment) (mg g−1) | 0.895 | 0.009 |
| Pseudo-second-order kinetic | ||
|---|---|---|
| R2 | 0.999 | 0.996 |
| K2 (g mg−1 min) | 0.034 | 0.0023 |
| qe (calculation) (mg g−1) | 0.96 | 0.0099 |
| qe (experiment) (mg g−1) | 0.895 | 0.009 |
| h0 (g mg−1 min) | 0.066 | 0.000 45 |
Note: R2 is correlation coeffecient, h0 is the initial adsorption rate.
Figure 12. Kinetic pseudo-first-orderof the adsorbtion of (a) pyrene (100 µg l−1), and (b) benzo(a)pyrene (1 µg l−1). Kinetic pseudo-seconde-order plote of (c) pyrene (100 µg l−1), and (d) benzo(a)pyrene (1 µg l−1) adsorption process on the surface of the green synthesized iron oxide nanoparticles, at iron oxide dose is 90 mg l−1, room temperture 20 °C and pH 7.
Download figure:
Standard image High-resolution image3.6. Isotherm investigation
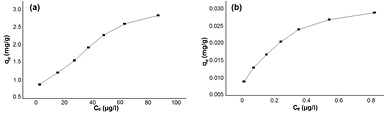
This study was carried out at different concentrations of pyrene and benzo(a)pyrene, where the equilibrium adsorption isotherm on the surface of the green synthesized IONPs was examined. This investigation was conducted under the optimized conditions of temperature, pH and the dose of the green synthesized IONPs. Figure 13 represents the isotherm plots of pyrene and benzo(a)pyren. The two isotherm models, namely, Langmuir (equation (9)) and Freundlich (equation (10)) were utilized for describing the experimental isotherm data:
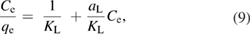
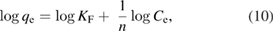
where qe (mg g−1) is the maximum adsorption capacity at equilibrium, Ce (mg l−1) is the residue concentration of pyrene or benzo(a)pyrene detected in the solution at equilibrium, and KL and aL (l g−1) are Langmuir constants. In equation (10) KF and n are the relative adsorption capacity constant and the adsorption intensity constant, respectively.
Figure 13. Isotherm plot of (a) pyrene (b) benzo(a)pyrene, at iron oxide dose of 90 mg l−1, room temperture, pH 7 and contact time 150 min.
Download figure:
Standard image High-resolution imageFigures 14(a) and (b) exhibit the Langmuir isotherm model plots of pyrene and benzo(a)pyrene, respectively. Meanwhile, figures 14(c) and (d) show the Freundlich isotherm model plots of pyrene and benzo(a)pyrene, respectively. The adsorption isotherm parameters of Langmuir and Freundlich isotherm models, for the adsorption of pyrene and benzo(a)pyrene, are given in table 6. In the present study Langmuir isotherm model plot of pyrene and benzo(a)pyrene are linear. This indicates that the Langmuir isotherm model fits the adsorption process better than Freundlich isotherm model.
Table 6. Adsorbtion isotherm parameters of the applied isotherm models.
| Isotherm models | Pyrene | Benzo(a)pyrene |
|---|---|---|
| Langmuir isotherm constant | ||
| R2 | 0.905 | 0.981 |
| KL(l g−1) | 0.162 | 0.337 |
| aL(l g−1) | 0.0483 | 10.7 |
| Freundlich isotherm constant | ||
|---|---|---|
| R2 | 0.895 | 0.97 |
| Kf (l g−1) | 0.63 | 0.031 |
| N | 3.14 | 3.56 |
Figure 14. Langmuir isotherm model plots of (a) pyrene, and (b) benzo(a)pyrene. Freundlich isotherm model plots of (c) pyrene, and (d) benzo(a)pyrene at iron oxide dose 90 mg l−1, room temperture, pH 7 and contact time 150 min.
Download figure:
Standard image High-resolution imageIt is worth mentioning that the classical Langmuir isotherm model is an equation with the best theoretical basis. It assumes that the adsorption is limited to one monolayer onto a surface containing a finite number of typical identical and homogeneous sites. It is, thus, assumed that molecules striking the surface have a given probability of adsorption. Consequently, molecules already adsorbed similarly should have a given probability of desorption. At equilibrium, therefore, equal numbers of molecules desorb and adsorb at any time. Such probabilities are related to the strength of the interaction between the adsorbent surface and the adsorbate. This classical model assumes uniform energies of adsorption onto the surface and assumes that there is no transmigration of the adsorbate on the surface plane [36, 37].
3.7. Regeneration of the iron oxide nanoparticles
One purpose of this study is to evaluate the possibility of regenerating and reuse of the green synthesized IONPs for the adsorption of pyrene and benzo(a)pyrene. For this purpose, the used IONPs were thoroughly washed with methanol. Methanol was selected due to the following advantages:
- Methanol solubility of pyrene and benzo(a)pyrene are 1 mg ml−1 and 0.1 mg ml−1, respectively.
- It is non toxic compared to other solvents (i.e. benzene, toluene, chloroform, etc).
- It is easily removed by rotary evaporator.
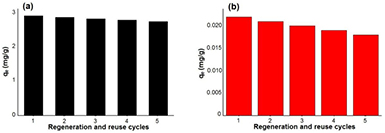
The capacities of the adsorption process of pyrene and benzo(a)pyrene by the regenerated IONPs, during the five times of regeneration and reuse, are shown in figure 15. It was noticed that there is no remarkable decrease in the adsorption capacity of the regenerated IONPs toward pyrene and benzo(a)pyrene up to five cycles of regeneration and reuse (i.e. the iron oxide nanoparticles are a potential candidate for the adsorption of pyrene and benzo(a)pyrene even for five cycles). This indicates the high applicability of the reuse of this green synthesized IONPs.
Figure 15. Adsorption of (a) pyrene and (b) benzo(a)pyrene onto the used green synthesized iron oxide nanoparticles surface.
Download figure:
Standard image High-resolution imageTable 7 shows that the green synthesized IONPs are efficient material for the adsorption of pyrene and benzo(a)pyrene from aqueous media and offers significant advantages over many of those previously described using traditional adsorbents.
Table 7. Different materials used for pyrene and benzo(a)pyrene adsorption.
| Adsorbent | Dose | Time of contact | Temperature (°C) | Removal capacity (%) | Ref. |
|---|---|---|---|---|---|
| Biochar formed at 400, 600 and 800°C | 1–8 g l−1 | — | — | 71.8–88.1% (at 400 °C), | [38] |
| 82.4–93.4% (at 600 °C), | |||||
| 95.8–98.6% (at 800 °C) | |||||
| PAC anthracite based and coconut shells based | 50 mg mixed with 40 ml of PAHs | 30 d | 25 °C | Pyrene: 98% | [39] |
| Benzo(a) pyrene: 88% | |||||
| Wood ashes | 10 mg ml−1 | 24 h | 25 °C | 100% for ashes obtain at 800 °C, | [40] |
| >99 for ashes obtained at 500 °C | |||||
| Leonardite (immature coal) | 0.5 g mixed with500 ml of PAHs | 24 h | 25 °C | Pyrene: 95%, | [41] |
| Benzo(a)pyrene: 88% | |||||
| Wood char | 5 mg l−1 | 9 d | 25 °C | ⩾60% | [42] |
| LSTR with PDLGA surfactant | 6 mg laccase and 150–160 mg LSTR mixed with 50 ml of PAHs | 24 h | — | 95.3–97.6% | [43] |
| Coke-derived porous carbon | 0.1 g mixed with 100 ml of PAHs | 7 h | 25 °C | >99% | [44] |
| Green synthesized IONPs | 90 mg l−1 | 150 min | — | Pyrene: 98% | This work |
| Benzo(a)pyrene: 99% |
PAC: powder activated carbon, LSTR: laccase loading spider type reactor, PDLGA: poly(D,–lactide coglycolide).
3.8. The semi pilot-plant for the removal of benzo(a)pyrene and pyrene from water
Table 8 gives the physical and chemical characteristics of the artificial contaminated tap water with the studied PAHs. Table 9 illustrated the results of the removal of pyrene and benzo(a)pyrene by using different doses of IONPs namely 90, 110 and 130 mg l−1, where the contact time was 150 min. The results showed that removal rate of pyrene and benzo(a)pyrene varied according to the implemented dose of the IONPs. These results indicated that the use of 110 mg l−1 of IONPs decreased the concentration of pyrene and benzo(a)pyrene from 100 and 1 µg l−1 to 1.7 and 0.02 µg l−1, respectively, and the corresponding rate of removal reached 98.3 and 98.0%, respectively. Also the use of 130 mg l−1 of IONPs decreased the concentration of pyrene and benzo(a)pyrene from 100 and 1 µg l−1 to 1.5 and 0.01 µg l−1, respectively, and the corresponding rate of removal reached 98.5 and 99.0%, respectively. Therefore, 130 mg l−1 of IONPs can be used as the optimum dose for the removal of pyrene and benzo(a)pyrene from drinking water. Thus it can be concluded that IONPs can be used to eliminate the PAHs in drinking contaminated water and can be employed in the dringking water treatment plants (DWTP) as a polishing tool.
Table 8. Physical and chemical characteristics of the artificial contaminated drinking water.
| Parameters | Artificial contaminated drinking water | |
|---|---|---|
| Maximum | Minimum | |
| pH | 7.33 | 7.10 |
| Temperature (°C) | 34 | 15 |
| Turburbidity (NTU) | 0.44 | 0.12 |
| Electric conductivity (µS) | 452 | 284 |
| Total dissolved solid (mg l−1) | 235 | 178 |
| Total suspended (mg l−1) | 239 | 142 |
| Chloride (mg l−1) | 22.9 | 20.4 |
| Residual chlorine (mg l−1) | 1.8 | 0.3 |
| Residual chlorine (mg l−1) | 135 | 99 |
| Ca-hardness (mg l−1) | 79 | 57 |
| Mg- hardness (mg l−1) | 67 | 40 |
| Nitrites (mg l−1) | 0.21 | 0.06 |
| Nitrate (mg l−1) | 0 | 0 |
| Phosphate (mg l−1) | 0 | 0 |
Table 9. Efficiency of the removal of PAHs from artificial contaminated table water by using iron oxide nanoparticles diferent doses and 150 min contact time.
| Parameter | Raw | Iron oxide nanoparticles dose (mg l−1) | |||||
|---|---|---|---|---|---|---|---|
| 90 | 110 | 130 | |||||
| C (µg l−1) | C (µg l−1) | R (%) | C (µg l−1) | R (%) | C (µg l−1) | R (%) | |
| Pyrene (µg l−1) | 100 | 2.1 | 97.9 | 1.7 | 98.3 | 1.5 | 98.5 |
| Benzo(a)pyrene (µg l−1) | 1 | 0.03 | 97 | 0.02 | 98 | 0.01 | 99 |
4. Conclusions
The pomegranate solid waste extract plays an important predominate role in the synthesis of iron oxide nanoparticles (IONPs), as an eco-friendly solvent. The physical characterization of green synthesized IONPs indicates that the amorphous Fe2O3 was obtained with an average particle size of 2.7 nm. Thus, it is classified as a powerful adsorbent.
The optimum conditions of the adsorption process at room temperature were pH 7, 150 min contact time, and optimum dose of 90 mg l−1. The adsorption capacity increases with increasing the initial concentration of pyrene and benzo(a)pyrene until reaching the equilibrium. The maximum adsorption capacities of pyrene and benzo(a)pyrene were found to be 2.8 and 0.029 mg g−1, respectively. Meanwhile, the total dissolved solids (TDS) of water have a positive effect on the adsorption process (i.e. the adsorption capacity increases by increasing the TDS of water).
Thermodynamic, kinetic, and isotherm studies show that the adsorption process of pyrene and benzo(a)pyrene by the green synthesized IONPs are exothermic, spontaneous in nature, chemisorption, and occurs on a monolayer of the adsorbent with homogeneous sites.
On the other hand, there is a high applicability of the regenerating and reusing of the green synthesized IONPs. Thus, the fresh and used IONPs adsorbents are potential candidates for the adsorption of pyrene and benzo(a)pyrene. The semi-pilot plant study exhibited efficient removal rate of the studied PAHs from artificial contaminated water. The rate of removal of pyrene and benzo(a)pyrene was 98.5 and 99%, respectively. This suggest that elimination of contaminated drinking water with PAHs can be successfully achieved by using IONPs as a polishing step in drinking water treatment plants.
Acknowledgment
The authors wish to express their deep appreciation and gratitude to the facilities provided by the project titled 'Sustainable Development for Wastewater Treatment and Reuse via Constructed Wetlands in Sinai' (SWWTR) that is funded by STDF of Egypt.