Abstract
This work focuses on the synthesis, functionalization, and characterization of magnetic nanoparticles to be used for improving the oil recovery in the oil exploitation industry. In this manuscript we explore three different types of hydrophobic/hydrophilic functionalization through a silanized particle: with styrene, with acrylic acid and with a copolymer of styrene and maleic acid. Further application of such nanoparticles dispersions (nanofluid) are discussed as the wetting and spreading behaviour of liquids on the solid surfaces change if the wettability of solid surface is altered. In order to investigate the influence of wettability alternation on enhancing oil recovery after nanofluid treatment, flushing oil experiment and contact angle measurement were conducted in our laboratory. The results indicated that nanofluid can produce a better flushing efficiency compared with brine solution, and the contact angles of oil phase increased from 13° to 37° after nanofluid treatment (0.005% w/w). We focus on the synthesis of magnetic iron oxide nanoparticles considering recovering possibility.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoparticles have attracted considerable attention in a wide range of disciplines, such as medical, environmental and petroleum industry applications [1–7]. Covalent functionalization is an important key to give stability to the material in drastic conditions such as high salinity, temperature, extreme pH etc. Since it is becoming increasingly difficult to discover new oilfields, the petroleum industry today is searching for new techniques to maximize the recovery factor and improve oil recovery. Functionalized nanoparticles seem to be interesting tools to achieve those goals.
In the past years, there have been several researches in nanoparticles applications in oil industry. In 2011, Ponnapati et al synthesized water-dispersible silica polymer nanoparticle hybrids for improving waterflood sweep efficiency. SiO2 ethylene oxide-based polymer nanoparticles hybrids were synthesized using a 'grafting-from' method, where polymer brushes were grown on silica nanoparticles using living radical polymerization. These nanohybrids did not yield high viscosity at concentrations in the range of 0.5–2 wt% [8]. Atta et al presented a magnetic powder based on magnetite nanoparticles, coated with rosin amidoxime, as a petroleum crude oil collector [9].
Sarmad et al examined how silica based nanofluids can induce such a wettability shift on oil-wet and mixed-wet calcite substrates. They analysed how pressure and temperature can significantly affect nanofluid properties [10]. Karimi et al investigated the effect of nanofluid of zirconium oxide on the wettability of carbonate samples [11]. In 2014 Mohammadi et al presented the effect of γ-Al2O3 on the wettability alteration of one of the Iran carbonate reservoirs. By injecting γ-Al2O3 nanofluid as a tertiary recovery, oil recovery increased by 11.25% [12].
In order to explore magnetic covalent functionalized nanoparticles behaviour for oil recovery we propose the following work. We focus on the synthesis of magnetic iron oxide nanoparticles considering recovering possibility. In this manuscript we explore three different types of hydrophobic/hydrophilic novel functionalization through particle silanization: with styrene, with acrylic acid and with a copolymer of styrene and maleic acid. Further application of such nanoparticles dispersions (nanofluid) in contact angle measurements, and the result of experiments using single glass capillaries to visualize the oil displacement process are discussed.
2. Experimental
2.1. Materials, crude oil and brine
All reagents and chemicals used in this study were of analytical grade. All inorganic and most of the organic reactants were provided by Merck Company. Acryloyl chlorhide was prepared from acrylic acid and thionyl chloride (Merck). Some specific reagents as triethylamine (TEA), petroleum ether, dimethylformamide (DMF) were purchased from Sintorgan S A. Azobisisobutyronitrile (AIBN) and γ-mercaptopropyl triethoxy silane (SPS) were provided by Teyupa S A.
A characterized sample of crude oil (26° API) was used for all the experiments without further treatment. The density of the crude was 0.90 g cm−3 at 25 °C and its viscosity was 1000 cp. Brine was prepared by dissolving 6.11 g of MgCl2·6H2O and 43.10 g of NaCl in 1 l of deionized water at 25 °C (pH = 6.5 ± 0.1).
2.1.1. Synthesis.
2.1.1.1. Magnetic nanoparticles.
For the synthesis of magnetic nanoparticles (MNs), a variation of a procedure reported by Vergés et al was used [13]. Shortly, two solutions were prepared, A and B. Solution A was prepared by dissolving 20.57 g of FeSO4·7H2O in 300 ml distilled water in a three-neck round bottom flask. The solution was heated with magnetic stirring to 80 °C for 30 min under an inert atmosphere of nitrogen. Solution B was prepared by dissolving 1.70 g of KNO3 and 14.36 g of KOH in 100 ml distilled water under nitrogen atmosphere for 5 min in a separate container. Then, solution B was slowly added to solution A reaching pH 12. After 45 min, the three-neck round bottom flask was cooled in the refrigerator. 1.5 h later, the system was placed on a magnet for 2 min, so the supernatant was discarded. The separated precipitate (black powder) was washed 4 times with distilled water, twice with ethanol and then with acetone. In each case, the suspension was centrifuged before discarding the supernatant. Finally, the precipitate was left to dry in a vacuum oven for 24 h at 30 °C.
2.1.1.2. Oleic acid coated iron oxide nanoparticles (MO).
A modification of a procedure reported by Bao et al was used for the synthesis of MNs, using oleic acid, as iron salts were dissolved in an acid solution and pH was carefully controlled during preparation [14]. First, 20 ml of HCl and 180 ml distilled water were mixed in a three-neck round bottom flask with 5.61 g of FeSO4·7H2O and the stoichiometric mass of FeCl3·6H2O (ratio Fe(III)/Fe(II)= 2/1), with magnetic stirring under an inert atmosphere of nitrogen. Then, ammonium hydroxide was quickly added to mixture under stirring at room temperature (pH = 9). After 30 min, oleic acid (5 ml) was continuously added into the mixture. The reaction temperature was raised to 91 °C in an hour. The necessary volume of HCl (0.01 M) was added in order to exclude base catalyst and unreacted oleic acid. The precipitate was collected by a magnetic field and washed several times by distilled water till the pH of the washing water was 7. Finally, the magnetic product was dried in an oven at 60 °C for 24 h.
2.1.2. Functionalization of MN.
For the functionalization of MNs, the procedure reported by Sun et al [15] was applied with some modifications. To synthesize the (3-acrylmercaptopropyl) triethoxy silane (AMPTS), a solution of SPS in toluene (1:10) was prepared and solid NaHCO3 was added, instead TEA used by Sun et al, giving a suspension, which was kept at 0 °C. On other hand, 0.065 ml of acryloyl chloride was dissolved with toluene at 0 °C, giving a solution, which was carefully added over the previous suspension in an ice-water bath with continuous stirring. The resulting mixture was magnetically stirred for 3 h at 0 °C, then kept 10 h at room temperature and the solid was filtered, giving AMPTS in solution. Then, 500 mg of MO were dispersed in 50 ml of toluene and sonicated during 1 h (instead 48 h a stirring at room temperature as described in literature) and mixed with AMPTS, toluene and TEA (2M in toluene). The mixture was stirred for 48 h at room temperature under nitrogen atmosphere. Thirdly, petroleum ether was added to the system to precipitate the mercaptoacrylic modified nanoparticles (MMNPs), followed by magnetic separation and dried in vacuum at 60 °C
2.1.2.1. Styrene (MS).
The functionalization with styrene was as follows: 3 ml of styrene and toluene were mixed in a round bottom flask with reflux. Then, 150 mg of MMNPs (prepared in section 2.1.2) and 20 mg of AIBN were added to the flask, and the system was sonicated during 10 min. The flask was immediately degassed by 2 cycles with N2, and then the mixture was magnetically stirred in a constant temperature sand bath at 80 °C during 4 h. Then, the system was washed 5 times with toluene using a magnet to separate the supernatant. The precipitate was left to dry in a vacuum oven at 50 °C for 24 h.
2.1.2.2. Acrylic acid (MA).
3 ml of toluene and 0.8 ml of acrylic acid were mixed in a round bottom flask. Then, a sample of MN (prepared in section 2.1.2) and 9 mg of AIBN were added to the flask, and the system was sonicated during 10 min. The flask was degassed by 2 cycles with N2, and then the mixture was magnetically stirred in a constant temperature sand bath at 80 °C during 4 h. Then, the system was washed 5 times with toluene using a magnet to separate the supernatant. The precipitate was left to dry in a vacuum oven at 50 °C for 24 h.
2.1.2.3. Styrene and maleic acid (MSM).
14 ml of DMF and 0.9 ml of styrene were mixed in a round bottom flask. Then, a sample of MN (prepared in section 2.1.2) and 9.4 mg of AIBN were added to the flask, and the system was sonicated during 10 min. The flask was degassed by 2 cycles with N2, and then the mixture was magnetically stirred in a constant temperature sand bath at 80 °C. After 1 h, maleic acid was added to the flask and the mixture was magnetically stirred in the sand bath during 3 h. Then, the system was washed 5 times: 2 times with DMF and 3 times with toluene, using a magnet to separate the supernatant. The precipitate was left to dry in a vacuum oven at 50 °C for 24 h. The samples nomination and description are shown in table 1.
Table 1. Samples nomination and description.
| Nomination | Description |
|---|---|
| MN | Magnetic nanoparticles as prepared |
| MO | Magnetic nanoparticles coated with oleic acid |
| MMNP | Magnetic nanoparticles coated with S-acryl groups |
| MS | Magnetic nanoparticles functionalized with styrene |
| MA | Magnetic nanoparticles functionalized with acrylic acid |
| MSM | Magnetic nanoparticles functionalized with styrene and maleic acid |
2.2. Nanofluid preparation
The preparation method of the nanofluid is a one-step process that consists of dispersing the dark particles in the fluid. In this work, a mass of magnetic nanoparticles (MN, MO, MS, MA and MSM) was dispersed in 20 ml of brine in order to obtain a light translucent solution (0.005% w/w). The nanofluid were sonicated during 16 h.
2.3. Characterization
The crystallinity of MN was studied using x-ray diffraction (XRD) with a Siemens XRD instrument. For structural characterization transmission electron microscopy (TEM) was performed. Hysteresis loops M versus H were measured with a maximum applied field of 15 kOe. Thermogravimetric (TG) analysis of the functionalized samples was carried on a thermal gravimetric analyser (Shimadzu, DTG-50) under a linear heating of 10 K min−1, in air atmosphere. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet FTIR Instrument 510P. For this purpose, KBr and powder samples pellets were prepared. All experiments in this study were performed at room temperature.
2.3.1. Measurement of contact angle.
Contact angle variation of pendant petroleum drops on a glass substrate was measured (figure 1). Once the drop-shape stopped changing, a digital camera (Sony—DSC-HX300 20.4-megapixel digital camera) was used to take the pictures. Two different size drops were selected (30 µl and 5 µl). The contact angle was obtained from the drop-shape analysis using Image J (National Institutes of Health). The experimental setup used is shown in figure 1.
Figure 1. (a) Experimental setup to measure the contact angle at 25 °C and (b) scheme showing the measured contact angle.
Download figure:
Standard image High-resolution imageTo decrease error, each experiment was replicated at least twice and the value was reported if minimum acceptable error was achieved.
2.3.2. Crude oil displacement from a glass capillary.
We conducted oil displacement tests from single glass capillaries (ID = 1166 µm—BioCap) to visualize the crude oil displacement process from the solid surface of the capillary, in the presence of brine and different nanofluid at 25 °C. The capillaries were placed vertically in oil, so oil was sucked into the capillaries under the capillary pressure, and filled with oil from the top. Then, the capillaries were immersed each inside different syringes filled with brine and nanofluid. The upper part of the capillaries was covered with oil to avoid a second meniscus in the capillary (figure 2). We compared oil displacement using different nanofluid prepared as in section 2.2.
Figure 2. Experimental setup of oil displacement using a glass capillary in the presence of different nanofluid.
Download figure:
Standard image High-resolution image3. Results and discussion
It is well known that magnetite (Fe3O4) nanoparticles can be easily oxidized by air oxidation and are easily aggregated in aqueous solutions due to anisotropic dipolar attraction, which restricts its applications [16], so surface modifications of magnetite using covalent bonding or physical coating can be used to avoid these processes. In these work all covalent functionalization were performed through the synthesis of silanized nanoparticles, having an acrylic terminal group, MMNPs, prepared from AMPTS and MO.
The following scheme (figure 3) represents the different steps described in section 2.1.2.
Figure 3. Scheme representing the functionalization steps: (a) from MO to MMNP, (b) from MMNP to MS, MA and MSM.
Download figure:
Standard image High-resolution image3.1. XRD analysis and magnetic characterization
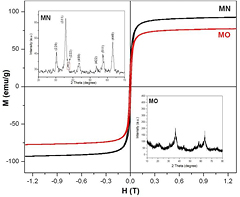
Figure 4 shows the magnetic profiles of MN and MO. Both samples present a superparamagnetic behavior with high magnetization values (Ms) and very low coercive field values (Hc). The inset of these samples shows a good spinel structure where all diffraction lines can be indexed with magnetite (or maghemite). This is related to their magnetic properties. When samples are coated with oleic acid, lower size nanoparticles are obtained with low crystallinity that slight diminishes the magnetic properties.
Figure 4. Magnetic profiles of the MN and the MO nanoparticles and their diffraction patterns.
Download figure:
Standard image High-resolution image3.2. Transmission electron microscopy analysis
Figure 5 shows TEM images from both samples MN and MO. We can appreciate a homogeneous size distribution near 15 nm.
Figure 5. TEM images show faced particles with homogeneous size distribution: (a) MN and (b) MO.
Download figure:
Standard image High-resolution image3.3. Thermogravimetric analysis
Taking into account that organic matter is much less stable than inorganic particles, thermogravimetric analysis (TGA) could be used to estimate the amount of organic mass in the functionalized material. As can be observed from figure 6, the loss of mass at 100 °C (attributable to water loss) is only significant for MSM (4.6%), because MS and MA only lose 1.7 and 1.9%.
Figure 6. Thermogravimetric profiles of the oleic coated and functionalized samples.
Download figure:
Standard image High-resolution imageThe thermal profile of MO is presented in order to show that oleic coating is present as this sample presents a loss of mass near 30%.
The shapes of the curves for MS and MA are very similar, and both show two stages decomposition. From 100 °C a slow mass falling begins, followed by a rapid loss of mass (13.3% between 350 to 490 °C for MS and 18.1% from 100 to 455 °C for MA). The second stage starts at 510 °C for MS and 455 °C for MA, and the total mass loss at 700 °C is 18.1% and 24.8% respectively. Even though the decomposition temperature may change with the molecular weight, polymers with aromatic pendant groups usually decompose at higher temperatures than the aliphatic ones. This fact is in agreement with the decomposition temperatures observed for MS and MA. For MSM, after the loss of water around 100 °C, a slow mass loss starts up to 285 °C, when a new decomposition event begins. This event represents de main loss of mass for MSM, and ends at 610 °C. The total mass loss for MSM until 700 °C was 41,4%.
As result of these analyses, we can estimate that the incorporation of organic material is 16.4% for MS, 22.9% for MA and 36.8% for MSM. Taking into account that all reaction time were the same and the availability of monomer was higher for MS than for MA and MSM, polymerization was more successful in the last cases, judging by the organic mass incorporated. These results could be attributed to the fact that polymerization can start within the solution, not only on the surface of the particle, so, the growing chain choose polymerize with the available monomer in solution rather than with the MMNP, due to the probable steric hindrance. If the availability of the monomer in solution decreases, then more monomer is incorporated into the nanoparticle.
3.4. Fourier transform infrared analysis
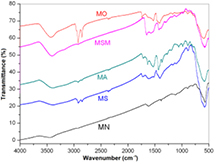
The modifications made on MNs were confirmed by FTIR analysis (figure 7). Spectrum of MN is very simple, two of the main absorptions are presented as a wide band between 3100 and 3660 cm−1 with a maximum absorption at 3440 cm−1 (stretching O-H), a medium absorption at 1617 cm−1 (scissoring H–O–H), both consistent with the presence of water. The mains adsorption peaks, according with literature [17], are the strong band at 573 cm−1 attributed to Fe–O vibration, meanwhile weak signals located at 890 and 800 cm−1 that can be assigned to superficial Fe–OH. All the samples show some strong bands around 600 cm−1 and the weak bands around 890 and 800 cm−1 (595, 790, and 892 cm−1 for MO; 582, 797, and 892 cm−1 for MS; 581, 794, and 889 cm−1 for MA; 580, 794, and 886 cm−1 for MSM), so we can assure that the functionalization does not cover the entire particle surface.
Figure 7. FTIR spectra of magnetic nanoparticles shown in table 1.
Download figure:
Standard image High-resolution imageMO, MS, MA and MSM, all showed strong evidences for the presence of the organic material, mainly by two absorption bands assigned to C–H stretching, which are present in all functionalized particles: 2926/2850 cm−1 for MO, 2919/2850 cm−1 for MS, 2919/2857 cm−1 for MA, and 2919/2857 cm−1 for MSM. They also showed a band for O–H stretching, due to the presence of carboxylic acids (3427 cm−1 for MO, 3400 cm−1 for MA, 3395 cm−1 for MSM) or residual hydroxyl groups (3405 cm−1 for MS).
For MO, absorptions at 3427and 1736 cm−1 indicated the presence of a carboxylic acid in their carbonyl form, like in the external part of oleic layer (figure 3), but also other two absorptions were seen at 1527 and 1425 cm−1, and they indicate the presence of oleic acid in their carboxilate form [18] as in the internal oleic shell, interacting by chemisorption with the nanoparticle surface. In addition, a weak absorption at 3005 cm−1 (stretching C=C–H) and a band at 1620 cm−1 (stretching C=C) confirm the presence of oleic acid.
Even when the absorption bands have low definition, indicating a disordered environment, MS shows absorptions compatibles with polystyrene structure (1650, 1581, and 1422 cm−1, aromatic C=C stretching vibration). Polystyrene typical bands at 695 and 765 cm−1 corresponding to C–H deformation of the monosubstituted aromatic ring were eclipsed in this spectrum by strong and wide absorption at 580 cm−1.
FTIR for MA derivative shows, in addition to the already mentioned absorptions, a band at 1718 cm−1, associated to C=O stretching, but also can be seen absorptions at 1533 and 1434 cm−1, suggesting that some of the carboxylic groups are partially neutralized. Other absorptions reported for polyacrylic acid [19] can be seen at 822 cm−1 (C–C stretching), 1060 cm−1 (C–O stretching), and 1271 cm−1 (C–C–O stretching).
For the case of MSM, some lack of definition, as in MS spectrum, could be observed, probably due to superposition of bands as well as to the disordered polymer chains. Nevertheless, the signal for C=O stretching at 1726 cm−1 for maleic residues is clearly observable, as well as some absorptions around 1600, 1550 and 1400 cm−1 compatibles with those observed for MS.
It is important to notice that, as the functionalization did not cover the entire surface, MS and MA showed an additional band at 984 cm−1 which could be assigned to Si–O–Si bond, assessing the surface silanization [20]. For MSM derivative a shoulder was detected at 985 cm−1, but could not be individualized as a single absorption.
3.5. Measurement of contact angle
Contact angle (CA) is the most universal measure of the wettability of surfaces: it quantifies the wettability of a substrate by a liquid. If the contact angle is small, it means that the drop is attracted to the solid. The increase in the contact angle means that the liquid becomes less attracted to the solid.
The results of contact angle measurements after the application of brine and nanofluid are shown in figure 8. These pictures and the results obtained from Image J show a contact angle alteration when the drop was introduced and stabilized in the brine and in the different nanofluid. The lower angle values belong to the oil drop immersed in brine. All the other samples present larger angles. Despite that, the angles using MA nanofluid are the lowest comparing with the other nanofluid. This functionalization was made in order to obtain a nanofluid with more affinity for the glass, which represents the substrate where oil is attached, based on the incorporation of hydrophilic functional groups. The modification increases the polarity, but also increases sample water 'solubility', so, the MA prefer stay in water suspension instead of contributing to the detachment of the drop of oil, and the contact angle shows the lower value for the studied nanofluid. As styrene is a hydrophobic chain, MS does not interact so much with water and its behaviour is similar than the observed for nude particles (MN). If MS interact with the oil drop, due to the lipophilicity of the polymer, these particles exert little effect on the oil-glass interaction. In literature [21] the detachment of oil drops with a nanofluid was described as an ordering of nanoparticles in layers between the solid and the drop, as a 'wedge'. Perhaps the change in the contact angle observed for MS and MN could be attributed to behaviour like that.
Figure 8. Contact angle test results for different solutions (0.005% w/w): Inlet: microphotograph of experimental measurement.
Download figure:
Standard image High-resolution imageAs the oil drop immersed in MSM ferrofluid presents the largest values of contact angle, we conclude that MSM nanofluid could be considered as the most effective fluid in increasing the contact angle as it includes both types (hydrophobic and hydrophilic) of monomer substitution, styrene and maleic acid respectively. According with de the methodology of synthesis, MSM particles have a hydrophobic core (build in polystyrene) surrounded by an hydrophilic exterior due to the poly (styrene-co-maleic acid). The effect of MSM on the contact angle could be attributed to its ability to interact with a polar substrate as the glass surface, due to its hydrophilic exterior. Since this behaviour was not observed for MA, there must be another reason to justify MSM performance. MA has only carboxylic acids as pendant groups in the chain; meanwhile MSM has a co-polymer, with hydrophobic-hydrophilic characteristics, like a detergent, and we think that is the reason for its outstanding performance. In that sense, recent literature mentions the use of styrene-maleic acid copolymers as an alternative to detergents for the solubilization, purification and characterization of integral membrane proteins [22].
Measurements for all nanofluid were performed on diluted samples (5 × 10−3% w/w) as we chose an optical method to determine the contact angle so, the obtained values are lower than those reported by other authors for other nanofluid [12, 21, 23].
Since the MSM nanofluid presents the largest contact angles, we decided to try with two different concentrations. The nanofluid, prepared as in section 2.2, results with C2 = 10 × C1 (0.05 and 0.005% w/w, respectively). Both nanofluid were sonicated during 16 h. To measure the contact angle, we proceeded as in section 2.3.1. Figure 9 shows the different shape of the drops: the oil drop immersed in C2 ferrofluid seems to be less attracted to the glass. Also, table 2 shows that the contact angle increases by increasing the MSM concentration.
Figure 9. Contact angle test results for different concentrations of MSM: (a) C1 and (b) C2.
Download figure:
Standard image High-resolution imageTable 2. Contact angle for C1 and C2.
| MSM (C1) | MSM (C2) | |
|---|---|---|
| Left angle (°) | −37.1 | −48.6 |
| Right angle (°) | −36.5 | −47.2 |
These results confirm that MSM effectively modifies the contact angle as function of its concentration. These experiments, which depend on visual observations, are limited by the opacity of the suspension, but they confirm the presence of MSM nanoparticles in the oil/glass interphase.
3.6. Crude oil displacement from a glass capillary
Figure 10 shows the capillaries immersed inside different syringes filled with brine and nanofluid, after 24 h. It is observed that more crude oil was displaced in the different nanofluid, compared to the brine (figure 10(a)). The same amount of oil was displaced using MN and ME nanofluid (figures 10(b) and (c)), and larger amount of crude oil was displaced using MA nanofluid (figure 10(d)). As seen from figure 10(e), MSM nanofluid displaced almost all the oil from the capillary. We conclude then that MSM nanofluid could be considered as the most effective of assayed fluid in displacing crude oil from a capillary.
Figure 10. Experimental setup of crude oil displacement from a single glass capillary (ID = 1166 µm) in the presence of different fluids at 25 °C: (a) Brine, (b) MN, (c) MS, (d) MA, (e) MSM.
Download figure:
Standard image High-resolution imageSince the MSM nanofluid displaced the largest amount of oil, we decided to compare the crude oil displacement at two different concentrations of MSM nanofluid (presented in section 3.5). Then, two syringes were filled with MSM (C1) and MSM (C2), respectively, and the capillaries filled with oil were immersed in each one. The oil displacement was observed as time goes on. Figure 11 shows that after 3 min, oil displacement started in MSM (C2), whereas it did not begin in MSM (C1). After 18 min, displacement begin in MSM (C1), but at the same time, the capillary immersed in MSM (C2) was almost empty. All the oil was recovered from capillary immersed in MSM (C2) after 20 min. After 2 h, there was still oil to be displaced in MSM (C1). We can conclude that the oil displacement (oil recovery) is faster as the nanofluid concentration increase.
Figure 11. Crude oil displacement from a glass capillary for two different nanofluid and brine solution versus time at 25 °C.
Download figure:
Standard image High-resolution imageIf we consider this ability for oil displacement in a capillary as a simplified model for the displacement effect of this nanofluid in porous rock material, we can conclude that its behavior is highly promissory for future field test.
4. Conclusions
MNs with attached polymers were prepared by applying radical polymerization on silanized MMNP's, and the resulting hybrids were brine-dispersible and stable. Is important to note that, as the functionalization did not cover the entire surface, de degree of functionalization could be increased in the future, and probably increase its effect for the same concentration. These nanohybrids did not yield high viscosity at concentrations in the range of 0.005–0.05 wt.%, so, they would pass easily and with low resistance through porous media.
Best results were achieved with MSM, which was synthesized using two different monomers, giving nanoparticles with an amphiphilic exterior, like a detergent. This amphiphilic quality gives emulsifying characteristics to nanofluid. Measurements of contact angle and oil displacement studies demonstrate that these nanohybrids are effective in the mobilization of waterflood residual oil.
Acknowledgments
This project was partially funded by UBACyT (2014–2017).