Abstract
In this study, silver nanoparticles (SNPs) were synthesised in an aqueous solution of corn starch. To fabricate the SNPs, reaction conditions, such as varying silver nitrate ( ) concentration, time, temperature and solution pH of the reaction, were optimized. Since, the optimum reaction conditions were found 1 mmo l−1, 15 min and
) concentration, time, temperature and solution pH of the reaction, were optimized. Since, the optimum reaction conditions were found 1 mmo l−1, 15 min and  , respectively. Then, to study the role of pH on SNP synthesis, varying pH values of the solution (3, 5, 7, 9 and 11) were investigated. Subsequently, the obtained silver/starch nanocomposites were characterised using different techniques. The x-ray diffraction (XRD) results revealed that the particles were face-centred cubic (FCC), and had an average particle size of 7.5 nm. This was confirmed by high-resolution transmission electron microscopy (HR-TEM) images. Moreover, the synthesised SNPs, at different pH values, were used as nanocatalyst for the reduction of 4-nitrophenol to 4-aminophenol in the presence of sodium borohydride. Under optimum reaction conditions, the higher catalytic activity was obtained with SNPs synthesised at pH 11 compared to lower pH of 7 or 9. Therefore, the rapid, reproducible, cost-effective silver/starch nanocomposite can be widely used for various applications such as drug manufacturing (e.g. analgesics and antipyretics) and the removal of pollutants from wastewater.
, respectively. Then, to study the role of pH on SNP synthesis, varying pH values of the solution (3, 5, 7, 9 and 11) were investigated. Subsequently, the obtained silver/starch nanocomposites were characterised using different techniques. The x-ray diffraction (XRD) results revealed that the particles were face-centred cubic (FCC), and had an average particle size of 7.5 nm. This was confirmed by high-resolution transmission electron microscopy (HR-TEM) images. Moreover, the synthesised SNPs, at different pH values, were used as nanocatalyst for the reduction of 4-nitrophenol to 4-aminophenol in the presence of sodium borohydride. Under optimum reaction conditions, the higher catalytic activity was obtained with SNPs synthesised at pH 11 compared to lower pH of 7 or 9. Therefore, the rapid, reproducible, cost-effective silver/starch nanocomposite can be widely used for various applications such as drug manufacturing (e.g. analgesics and antipyretics) and the removal of pollutants from wastewater.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Green chemistry is a growing branch of nanotechnology that aids in the removal of environmental organic pollutants. Organic pollutants such as nitrophenols (NPs), polychlorinated biphenyls and organochlorine pesticides are frequently found in industrial and agricultural wastewater [1]. NPs (such as 2-NP or 4-NP and 2,4-diNP) are aromatic phenolic compounds that are used to manufacture dyes, insecticides, fungicides and drugs (such as acetaminophen) [2]. To remove organic pollutants [3], the processes such as electrochemical treatments, adsorption, the electro-Fenton method [4] and catalytic degradation techniques [5], are widely used. In 2002, Pradhan et al [6] for the first time performed the catalytic degradation of 4-NP to 4-aminophenol (4-AP). Further research since then has shown that sodium borohydride ( ) can only be used for the reduction of 4-NP [7]; however, this reaction was found to be inadequate in general. It is more favourable and appropriate to conduct this catalytic reduction in the presence of immobilised NPs as a catalyst [8].
) can only be used for the reduction of 4-NP [7]; however, this reaction was found to be inadequate in general. It is more favourable and appropriate to conduct this catalytic reduction in the presence of immobilised NPs as a catalyst [8].
In contrast to 4-NP, 4-AP, an intermediate chemical product in the catalytic reduction reaction, can be potentially used in many analgesics and fever-reducing drugs (such as paracetamol and phenacetin).
Noble metal NPs, such as platinum [2], gold [9, 10] and silver [11–14], are used as nanocatalysts for the reduction of 4-NP to 4-AP in the presence of excessive  . In the recent time, much attention has been focused on the green synthesis of silver nanoparticles (SNPs); therefore, we biosynthesised SNPs using plant leaf broth [15], beet root extract [16], Salmalia malabarica gum [17], chitosan [18], cellulose [19] and starch [20].
. In the recent time, much attention has been focused on the green synthesis of silver nanoparticles (SNPs); therefore, we biosynthesised SNPs using plant leaf broth [15], beet root extract [16], Salmalia malabarica gum [17], chitosan [18], cellulose [19] and starch [20].
There are many types of starch that are derived from potato, and other cereal starches such as corn, wheat, and rice sources. Starch, a natural hydrophilic polysaccharide polymer, is biodegradable and non-toxic and has low production costs. It is a semi-crystalline biopolymer that is both crystalline and amorphous in nature. The crystalline regions are primarily composed of an amylopectin polymer, in which the outer hydrogen branches are bound together to form crystallites. On the other hand, other amorphous regions of starch granules are chiefly composed of either amylose or amylopectin segments. Corn starch (CS) is abundant with amylose (approximately  ), and amylopectin (
), and amylopectin ( ) content [21]. It is used widely in various industries such as paper, binders, pharmacological, and textile industries. It can also be used as an alternative to other traditional plastics in the global markets. It was reported that the degree of starch granule disruption increases with increasing temperature above the gelatinisation temperature [22]. It was found that the CS gelatinisation temperature is around
) content [21]. It is used widely in various industries such as paper, binders, pharmacological, and textile industries. It can also be used as an alternative to other traditional plastics in the global markets. It was reported that the degree of starch granule disruption increases with increasing temperature above the gelatinisation temperature [22]. It was found that the CS gelatinisation temperature is around  [23], therefore it is expected that elevation of the heating temperature of CS will enhance its behavior by disruption of the starch granules and emergence of the starch contents. Also, CS with high dextrose content was obtained during starch hydrolysis compared to other starch sources (mung bean, sago, and potato) [23]. Moreover the presence of alkaline medium, at high elevated temperature, promotes and facilitates the reduction of metallic ions (e.g. Ag, Au, and Cu), as the glucose itself cannot reduce them [24, 25].
[23], therefore it is expected that elevation of the heating temperature of CS will enhance its behavior by disruption of the starch granules and emergence of the starch contents. Also, CS with high dextrose content was obtained during starch hydrolysis compared to other starch sources (mung bean, sago, and potato) [23]. Moreover the presence of alkaline medium, at high elevated temperature, promotes and facilitates the reduction of metallic ions (e.g. Ag, Au, and Cu), as the glucose itself cannot reduce them [24, 25].
Chairman and Somsook [26] and Valencia et al [27] synthesised SNPs using starch as a template; however, the fabrication process was very time consuming (7 d and 12 h, respectively). Furthermore, Sibiya, Xaba and Moloto [28] prepared SNPs using various starch concentrations ( ,
,  and
and  [w/v]), but the silver nitrate concentration (
[w/v]), but the silver nitrate concentration ( 0.1 M) used could be considered toxic to biomedical applications [29]. Up to the best of the author knowledge, it is the first time that controlling and optimizing the physical parameters of CS matrix preparation is to reduce and stabilise SNPs. This would enhance the catalytic reduction of 4-NP to 4-AP via Ag/starch nanocomposite at very high pH value. Therefore, this study aimed at synthesising SNPs using a rapid, green and simple approach. This was achieved by optimizing different physical reaction conditions, such as time, temperature, and solution pH of the reaction. Moreover, corn starch was used as both a reducing agent to reduce
0.1 M) used could be considered toxic to biomedical applications [29]. Up to the best of the author knowledge, it is the first time that controlling and optimizing the physical parameters of CS matrix preparation is to reduce and stabilise SNPs. This would enhance the catalytic reduction of 4-NP to 4-AP via Ag/starch nanocomposite at very high pH value. Therefore, this study aimed at synthesising SNPs using a rapid, green and simple approach. This was achieved by optimizing different physical reaction conditions, such as time, temperature, and solution pH of the reaction. Moreover, corn starch was used as both a reducing agent to reduce  colloidal solution and stabilising (capping) agent to protect the fabricated SNPs from aggregation and/or agglomeration.
colloidal solution and stabilising (capping) agent to protect the fabricated SNPs from aggregation and/or agglomeration.
2. Materials and methods
2.1. Materials
Regular corn starch containing approximately 74% amylopectin and 26% amylose, silver nitrate  (>
(> ), sodium hydroxide (NaOH), sodium borohydride (
), sodium hydroxide (NaOH), sodium borohydride ( ) and 4-nitrophenol (4-NP) were purchased from Sigma–Aldrich (St. Louis, USA). The glassware was washed with
) and 4-nitrophenol (4-NP) were purchased from Sigma–Aldrich (St. Louis, USA). The glassware was washed with  (3:1) mixture and subsequently with highly purified distilled water
(3:1) mixture and subsequently with highly purified distilled water  .
.
2.2. Synthesis of SNPs by using the CS solution
Weighted amount (1 g) of CS powder was dissolved in 100 ml of distilled water to prepare 1% (wt%) solution in a hot water ( ) with stirring at certain intervals to ensure complete dissolution of CS. The different concentrations of
) with stirring at certain intervals to ensure complete dissolution of CS. The different concentrations of  (0.1, 1, 10 and 100 mmol l−1) solution were prepared. The efficiency and the synthesis of SNPs in the biopolymer matrix were investigated under various experimental conditions by varying the
(0.1, 1, 10 and 100 mmol l−1) solution were prepared. The efficiency and the synthesis of SNPs in the biopolymer matrix were investigated under various experimental conditions by varying the  concentration, changing the temperature of the reaction and changing the reaction time for the reactants. These factors possibly play an important role in the fabrication of NPs and affect the size, shape and particle distribution.
concentration, changing the temperature of the reaction and changing the reaction time for the reactants. These factors possibly play an important role in the fabrication of NPs and affect the size, shape and particle distribution.
2.3. Characterization techniques of the synthesised SNPs
The absorption spectra of the prepared samples were measured by UV-visible (UV-Vis) using a spectrophotometer (Thermo Scientific Evolution 201 UV-vis, USA) that scanned in a wavelength range of  . Transmission electron microscopy (TEM) images were taken using JEOL TEM-210 model, which operates at 120 kV to study the size, morphology, and shape of the synthesised SNPs. Elemental composition analysis of SNP samples was carried out using an SEM (JOEL Model JED-2300, USA) supplied with an energy dispersive spectrometer detector. The x-ray diffraction (XRD) measurement was performed on x-ray diffractometer (Panalytical X'Pert Pro 3050/60) working at 30 kV and 100 mA and spectrum was recorded by Cu Kα radiation
. Transmission electron microscopy (TEM) images were taken using JEOL TEM-210 model, which operates at 120 kV to study the size, morphology, and shape of the synthesised SNPs. Elemental composition analysis of SNP samples was carried out using an SEM (JOEL Model JED-2300, USA) supplied with an energy dispersive spectrometer detector. The x-ray diffraction (XRD) measurement was performed on x-ray diffractometer (Panalytical X'Pert Pro 3050/60) working at 30 kV and 100 mA and spectrum was recorded by Cu Kα radiation  in the range
in the range  to
to  . Fourier transform infrared (FTIR) transmittance spectra of silver/starch nanocomposite powder were recorded at room temperature, using FT-IR spectrometer (Thermo Fisher Nicolet iS10, USA) in the range of
. Fourier transform infrared (FTIR) transmittance spectra of silver/starch nanocomposite powder were recorded at room temperature, using FT-IR spectrometer (Thermo Fisher Nicolet iS10, USA) in the range of  and using KBr pellets. Zeta potential measurements were carried out using Malvern instruments.
and using KBr pellets. Zeta potential measurements were carried out using Malvern instruments.
2.4. The catalytic reduction of 4-NP by silver/starch nanocomposite
The catalytic activity of CS-SNPs was investigated by examining the reduction of 4-NP in 4-aminophenol (4-AP) in the presence of ice cold freshly prepared  which acts as the reducing agent [12, 14, 30]. To estimate (assess) the catalytic reduction of 4-NP, 2 ml of 4-NP
which acts as the reducing agent [12, 14, 30]. To estimate (assess) the catalytic reduction of 4-NP, 2 ml of 4-NP  was added to
was added to  of
of  (
( ) in a quartz cuvette of 1 cm path length. This was followed by the addition of
) in a quartz cuvette of 1 cm path length. This was followed by the addition of  of CS-SNP different silver/starch nanocomposite solution samples and mixed well quickly. The reaction was monitored by UV-vis spectrophotometer by recording the absorption spectra. All experiments were carried out for various silver/starch nanocomposite samples under ambient conditions at room temperature
of CS-SNP different silver/starch nanocomposite solution samples and mixed well quickly. The reaction was monitored by UV-vis spectrophotometer by recording the absorption spectra. All experiments were carried out for various silver/starch nanocomposite samples under ambient conditions at room temperature  .
.
3. Results and discussion
3.1. Ultraviolet-visible and transmission electron microscopy studies
3.1.1. Effect of silver nitrate concentration and temperature on the synthesis of silver nanoparticles.
For the synthesis of SNPs, 25 ml of the previously prepared CS colloidal solution ( ) was mixed with 5 ml of
) was mixed with 5 ml of  (0.1, 1, 10 and 100 mmol l−1) in a basic pH medium for certain reaction time. Figure 1(a) shows the ultraviolet-visible (UV-vis) spectra of the absorbance intensity against various
(0.1, 1, 10 and 100 mmol l−1) in a basic pH medium for certain reaction time. Figure 1(a) shows the ultraviolet-visible (UV-vis) spectra of the absorbance intensity against various  concentrations. At 0.1 mmol l−1, there was no observable localised surface plasmon resonance (LSPR) peak, which is a characteristic peak that occurs when the electrons in the nanoparticles interact with the electromagnetic radiation. In general, the noble metal nanoparticles such as silver and gold afford strong extinction and scattering spectra due to such property [31].
concentrations. At 0.1 mmol l−1, there was no observable localised surface plasmon resonance (LSPR) peak, which is a characteristic peak that occurs when the electrons in the nanoparticles interact with the electromagnetic radiation. In general, the noble metal nanoparticles such as silver and gold afford strong extinction and scattering spectra due to such property [31].
Figure 1. UV-vis absorption spectra showing (a) the variation in the absorbance intensity at different  concentrations (0.1, 1, 10, and 100 mmol l−1) in the presence of CS (10 g l−1) for 15 min at
concentrations (0.1, 1, 10, and 100 mmol l−1) in the presence of CS (10 g l−1) for 15 min at  and (b) UV-vis spectrum of SNP solution at 1 mmol l−1
and (b) UV-vis spectrum of SNP solution at 1 mmol l−1  in the presence of CS (10 g l−1) as a function of temperature (50, 60, 70, 80, and
in the presence of CS (10 g l−1) as a function of temperature (50, 60, 70, 80, and  ) for 15 min (inset shows different samples at different temperatures).
) for 15 min (inset shows different samples at different temperatures).
Download figure:
Standard image High-resolution imageAccording to the Mie theory, the position of the UV-vis spectrum absorption peak directly depends on the size of the synthesised nanoparticles as follows:
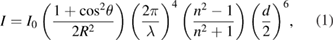
where  is the distance between the particles and the observer,
is the distance between the particles and the observer,  is the diameter of the particles,
is the diameter of the particles,  is the scattering angle and n is the refractive index of the particles [31–33].
is the scattering angle and n is the refractive index of the particles [31–33].
The absorbance intensity increased with the increasing  concentrations (1, 10, and 100 mmol l−1). The LSPR peak at the maximum wavelength (
concentrations (1, 10, and 100 mmol l−1). The LSPR peak at the maximum wavelength ( ) for 1 mmol l−1 and 10 mmol l−1 was 414 and 405 nm, respectively, whereas a slight red shift of the LSPR peak (
) for 1 mmol l−1 and 10 mmol l−1 was 414 and 405 nm, respectively, whereas a slight red shift of the LSPR peak ( ) was detected at 100 mmol l−1, possibly due to the formation of the large particles that may lead to the agglomeration and/or aggregation of the particles due to the supersaturation of the
) was detected at 100 mmol l−1, possibly due to the formation of the large particles that may lead to the agglomeration and/or aggregation of the particles due to the supersaturation of the  concentration [34].
concentration [34].
Figure 1(b) shows the UV-vis absorption spectra of SNPs capped by CS at different temperatures (50, 60, 70, 80 and  ) and higher pH for a certain interval. The absorption intensity increased with the increasing reaction temperature. Furthermore, the colour of the solutions gradually changed with a fixed LSPR peak at
) and higher pH for a certain interval. The absorption intensity increased with the increasing reaction temperature. Furthermore, the colour of the solutions gradually changed with a fixed LSPR peak at  . However, at
. However, at  , there was a blue shift of the LSPR peak (
, there was a blue shift of the LSPR peak ( ), indicating the formation of small nanoparticles [23, 35, 36]. Also, the colour change of the synthesised SNP samples at different temperatures was observed in the inset in figure 1(a)(i)–(v)). Hence, the gradual change of the colour, indicates the formation of the SNPs due to the reduction of
), indicating the formation of small nanoparticles [23, 35, 36]. Also, the colour change of the synthesised SNP samples at different temperatures was observed in the inset in figure 1(a)(i)–(v)). Hence, the gradual change of the colour, indicates the formation of the SNPs due to the reduction of  ions. Since, it is known that polysaccharides such as CS could be hydrolyzed by an amylase enzyme or by heat treatment at high temperature of
ions. Since, it is known that polysaccharides such as CS could be hydrolyzed by an amylase enzyme or by heat treatment at high temperature of  or higher [31]. Since the increase of the reaction temperature from
or higher [31]. Since the increase of the reaction temperature from  to
to  , that will enhance the amylose crystalline helical structure content to leach from the amorphous amylopectin of CS molecules [27, 37]. Whereas, this reaction could not complete without the heat supply. Also, the reaction will be optimized at the very high alkaline basic medium (more than pH 9) [38, 39].
, that will enhance the amylose crystalline helical structure content to leach from the amorphous amylopectin of CS molecules [27, 37]. Whereas, this reaction could not complete without the heat supply. Also, the reaction will be optimized at the very high alkaline basic medium (more than pH 9) [38, 39].
3.1.2. Effect of the pH of the reaction medium on the synthesis of silver nanoparticles.
To demonstrate the role of different pH on the synthesis of SNPs, 25 ml of corn starch solution was mixed with 5 ml of  (optimum suitable concentration), which was added drop wise at a hot plate at
(optimum suitable concentration), which was added drop wise at a hot plate at  with continuous stirring. Simultaneously, the pH of the mixture was adjusted to 3, 5, 7, 9 and 11 with a few drops of a freshly prepared 0.1 N HCl or 0.1 N NaOH solution.
with continuous stirring. Simultaneously, the pH of the mixture was adjusted to 3, 5, 7, 9 and 11 with a few drops of a freshly prepared 0.1 N HCl or 0.1 N NaOH solution.
According to the UV-vis absorption spectrum shown in figure 2(a), there was no change in the solution colour at low pH (3–7) compared to the blank sample (pure CS), indicating that SNPs were not formed. However, by increasing the pH (9–11), both the colour and the LSPR peak of the mixtures changed (figure 2(a), inset (i)–(v)) from faint yellow and  (pH 9) to deep yellow colour and
(pH 9) to deep yellow colour and  (pH 11), indicating that the SNPs were formed (figure 2(b)). It was reported by
(pH 11), indicating that the SNPs were formed (figure 2(b)). It was reported by  . Uthumporn et al [31] that, the gelatinization process of corn CS was achieved at
. Uthumporn et al [31] that, the gelatinization process of corn CS was achieved at  , therefore the heating of the CS at
, therefore the heating of the CS at  could enhance and improve the hydrolysis of CS amylose content to maltose molecules. Then, after the addition of NaOH which dissociates into sodium ions (
could enhance and improve the hydrolysis of CS amylose content to maltose molecules. Then, after the addition of NaOH which dissociates into sodium ions ( ) and hydroxyl ions (
) and hydroxyl ions ( ), these
), these  ions oxidise the CS molecules and generate heat. This will be followed by the dissociation of oxidized CS molecules into glucose units, whereas this reaction is enhanced in the presence of both high temperature (more than
ions oxidise the CS molecules and generate heat. This will be followed by the dissociation of oxidized CS molecules into glucose units, whereas this reaction is enhanced in the presence of both high temperature (more than  ) and very high alkaline basic reaction medium (i.e. pH 11). The reduction of Ag+ ions was done by the glucose units which contain a carbonyl group that named aldehyde, that is responsible for the reduction of
) and very high alkaline basic reaction medium (i.e. pH 11). The reduction of Ag+ ions was done by the glucose units which contain a carbonyl group that named aldehyde, that is responsible for the reduction of  ions to SNPs (
ions to SNPs ( ) [38]. This is consistent with the mechanism proposed by Shervani and Yamamto [25], where Ag2O molecules dissociate into silver ions (
) [38]. This is consistent with the mechanism proposed by Shervani and Yamamto [25], where Ag2O molecules dissociate into silver ions ( ), which subsequently get reduced to SNPs (
), which subsequently get reduced to SNPs ( ) in the presences of the electron pairs produced from CS fragment oxidation. In addition, the synthesised NPs could be stabilised by the helical amylopectin residue of CS molecules as shown in the proposed reaction mechanism in figure 3 forming Ag/CS nanocomposite. Hence, the CS acts not only as a reducing agent in an alkaline medium, but also as a capping (stabilising) agent.
) in the presences of the electron pairs produced from CS fragment oxidation. In addition, the synthesised NPs could be stabilised by the helical amylopectin residue of CS molecules as shown in the proposed reaction mechanism in figure 3 forming Ag/CS nanocomposite. Hence, the CS acts not only as a reducing agent in an alkaline medium, but also as a capping (stabilising) agent.
Figure 2. UV-vis absorption spectra of SNP solution (a) prepared at different pH values using 1 mmol l−1  in the presence of CS (10 g l−1) for 15 min at
in the presence of CS (10 g l−1) for 15 min at  ; (b) a plot of pH versus absorbance peak at different pH values (3, 5, 7, 9, and 11) of the medium.
; (b) a plot of pH versus absorbance peak at different pH values (3, 5, 7, 9, and 11) of the medium.
Download figure:
Standard image High-resolution imageFigure 3. The proposed mechanism of SNPs capped by CS using 1 mmole l−1  in the presence of CS (10 g l−1) for 15 min at
in the presence of CS (10 g l−1) for 15 min at  and pH 11.
and pH 11.
Download figure:
Standard image High-resolution image3.1.3. Effect of reaction time on the synthesis of silver nanoparticles.
The effect of the reaction time on the synthesis of SNPs, the reaction was carried out at different reaction time durations (15, 30, 45, 60 and 90 min). Then, the samples from the reaction medium were withdrawn at different time intervals, followed by recording the UV-vis absorption spectra of the fabricated SNPs. Figure 4(a) shows a gradual increase in the absorption intensity at different time intervals; the LSPR peak was observed at  . Furthermore, the calculation of the full width at half maximum (FWHM) of the recording data indicates that the FWHM gradually decreases with the increasing reaction time. In addition, the FWHM value at the lowest reaction time interval (15 min) was 76 nm, whereas it was 110 nm at the highest reaction time interval (i.e. 90 min) [figure 4(b)]. This could be correlated to the dependence of SNP synthesis on the reaction time interval [40]. However, using NaOH to elevate the pH of the medium to 11 accelerates the reduction of silver salt under different conditions and promotes the capping of the synthesised SNPs by CS fragments. Therefore, the reaction is carried out under different physical conditions to obtain smaller SNPs capped by a biopolymeric CS matrix [41, 42].
. Furthermore, the calculation of the full width at half maximum (FWHM) of the recording data indicates that the FWHM gradually decreases with the increasing reaction time. In addition, the FWHM value at the lowest reaction time interval (15 min) was 76 nm, whereas it was 110 nm at the highest reaction time interval (i.e. 90 min) [figure 4(b)]. This could be correlated to the dependence of SNP synthesis on the reaction time interval [40]. However, using NaOH to elevate the pH of the medium to 11 accelerates the reduction of silver salt under different conditions and promotes the capping of the synthesised SNPs by CS fragments. Therefore, the reaction is carried out under different physical conditions to obtain smaller SNPs capped by a biopolymeric CS matrix [41, 42].
Figure 4. UV-vis absorption spectra of the SNP solution (a) prepared at the different reaction time (15, 30, 45, 60 and 90 min) using 1 mmol l−1  in the presence of CS (10 g l−1) at
in the presence of CS (10 g l−1) at  , (b) plot of absorbance intensity at
, (b) plot of absorbance intensity at  and the FWHM of the absorption spectra versus different reaction time intervals.
and the FWHM of the absorption spectra versus different reaction time intervals.
Download figure:
Standard image High-resolution imageHigh resolution (HR) transmission electron microscopy (TEM) measurements showed that the synthesised SNPs capped by corn starch were bigger in size ( ; average particle diameter:
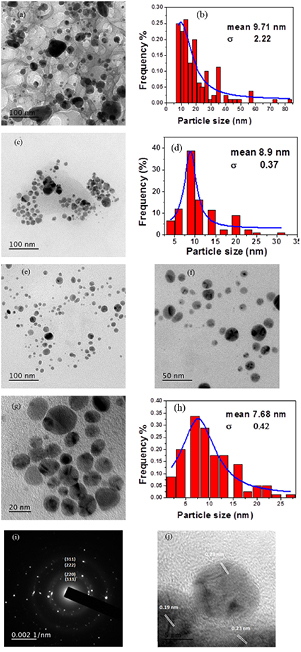
; average particle diameter: ) at pH 7 (figure 5), while at high pH (9 and 11), the SNPs were smaller in size (average particle size diameter: 8.9 and 7.68 nm, respectively). The spherical particles and the particle size distribution were relatively homogeneous for alkaline pH (pH 11), which is inconsistent with the UV-vis spectra [42]. Moreover, the selected area electron diffraction (SAED) image of the sample shows the concentric circles of diffraction pattern indicating that the obtained SNPs is highly crystalline [figure 5(i)]. In addition to, the HR-TEM images ascribes the space lattice of the metallic SNPs with 0.19 and 0.23 nm as shown in figure 5(j). Therefore, the TEM images confirm that the synthesised SNPs are metallic in nature.
) at pH 7 (figure 5), while at high pH (9 and 11), the SNPs were smaller in size (average particle size diameter: 8.9 and 7.68 nm, respectively). The spherical particles and the particle size distribution were relatively homogeneous for alkaline pH (pH 11), which is inconsistent with the UV-vis spectra [42]. Moreover, the selected area electron diffraction (SAED) image of the sample shows the concentric circles of diffraction pattern indicating that the obtained SNPs is highly crystalline [figure 5(i)]. In addition to, the HR-TEM images ascribes the space lattice of the metallic SNPs with 0.19 and 0.23 nm as shown in figure 5(j). Therefore, the TEM images confirm that the synthesised SNPs are metallic in nature.
Figure 5. TEM images and the histograms of SNPs prepared with 1 mmol l−1  in the presence of CS (10 g l−1) at
in the presence of CS (10 g l−1) at  for 15 min at pH 7 (a) and (b), pH 9 (c) and (d) and pH 11 at different levels of magnification (e)–(h). Selected area electron diffraction (SAED) pattern of SNPs (i) and HR-TEM image (j).
for 15 min at pH 7 (a) and (b), pH 9 (c) and (d) and pH 11 at different levels of magnification (e)–(h). Selected area electron diffraction (SAED) pattern of SNPs (i) and HR-TEM image (j).
Download figure:
Standard image High-resolution image3.2. X-ray diffraction (XRD) studies and energy dispersive x-ray (EDX) spectroscopy analysis
X-ray diffraction (XRD) pattern depicted three distinct, intense peaks, in the diffraction spectrum of 2θ, ranging from  to
to  . XRD pattern of SNPs stabilised by CS (10 mmol l−1) was characterised by three distinct, intense peaks at
. XRD pattern of SNPs stabilised by CS (10 mmol l−1) was characterised by three distinct, intense peaks at  ,
,  and
and  , representing the characteristic diffraction peaks of SNPs that were indexed to (111), (220) and (311) crystalline planes of the face centred cubic crystalline structure of metallic silver, respectively [figure 6(a)]. These results are inconsistent with an XRD spectrum of pure crystalline silver structure that has been published by the joint committee on powder diffraction standard (JCPDS file No. 00-004-0783) [43]. Furthermore, the average particle size can be estimated using the Scherrer formula:
, representing the characteristic diffraction peaks of SNPs that were indexed to (111), (220) and (311) crystalline planes of the face centred cubic crystalline structure of metallic silver, respectively [figure 6(a)]. These results are inconsistent with an XRD spectrum of pure crystalline silver structure that has been published by the joint committee on powder diffraction standard (JCPDS file No. 00-004-0783) [43]. Furthermore, the average particle size can be estimated using the Scherrer formula:

where  is the particle diameter (nm),
is the particle diameter (nm),  is a constant (a shape factor) that equals to 0.9,
is a constant (a shape factor) that equals to 0.9,  is the wavelength of the x-ray diffraction source (0.1541 nm),
is the wavelength of the x-ray diffraction source (0.1541 nm),  is the full width at half maximum and
is the full width at half maximum and  is the half diffraction angle (in degrees). Therefore, the average particle size diameter was calculated from the most predominant intense diffraction peak at
is the half diffraction angle (in degrees). Therefore, the average particle size diameter was calculated from the most predominant intense diffraction peak at  [44]. Thus, the calculated average particle size diameter was 7.31 nm, which is very close to that obtained from the TEM histogram distribution [45].
[44]. Thus, the calculated average particle size diameter was 7.31 nm, which is very close to that obtained from the TEM histogram distribution [45].
Figure 6. XRD of SNPs capped by CS (a) and (b) microanalysis EDX results of SNPs capped by CS.
Download figure:
Standard image High-resolution imageFigure 6(b) shows a characteristic peak around 3.0 keV, which corresponds to the binding energy of  , and other peaks near
, and other peaks near  , which correlate to the presence of C, O and Na impurities. The EDX spectrum confirms the presence of SNPs [46, 47].
, which correlate to the presence of C, O and Na impurities. The EDX spectrum confirms the presence of SNPs [46, 47].
3.3. Fourier transformation infrared (FT-IR) spectroscopy studies and zeta potential of silver nanoparticles capped by CS
The structural changes due to the interaction between the starch molecules and synthesised SNPs were investigated by using Fourier transformation infrared (FT-IR) spectra. Figure 7(a) shows the FT-IR absorption spectra of native starch and SNP/starch nanocomposite. A strong absorption band at  was attributed to the O-H stretching of starch. In addition, a sharp band of asymmetric stretching of the C-H band was assigned at
was attributed to the O-H stretching of starch. In addition, a sharp band of asymmetric stretching of the C-H band was assigned at  . Moreover, a strong band observed at
. Moreover, a strong band observed at  was attributed to the water adsorbed in the amorphous amylose region of starch [48]. The characteristic peaks at 1085 and
was attributed to the water adsorbed in the amorphous amylose region of starch [48]. The characteristic peaks at 1085 and  were due to the C-O-H bending of starch molecules and anhydroglucose ring O-C stretch, respectively [49]. The shift at
were due to the C-O-H bending of starch molecules and anhydroglucose ring O-C stretch, respectively [49]. The shift at  and
and  bands to a lower wavelength at
bands to a lower wavelength at  and
and  in the presence of SNPs was possibly due to the interaction of SNPs with the OH groups of SNP/CS nanocomposites. Furthermore, the other two characteristic bands at 1085 and
in the presence of SNPs was possibly due to the interaction of SNPs with the OH groups of SNP/CS nanocomposites. Furthermore, the other two characteristic bands at 1085 and  shifted to 1076 and
shifted to 1076 and  , respectively, due to the coordination between the oxygen atom of the starch OH polar groups and Ag ions [47].
, respectively, due to the coordination between the oxygen atom of the starch OH polar groups and Ag ions [47].
Figure 7. FTIR spectra of pure starch and SNPs capped by CS (a) and (b) the average values of zeta potential for SNPs capped by CS.
Download figure:
Standard image High-resolution imageFigure 7(b) represents the ZP measurements of SNPs stabilised by corn starch. The ZP of the SNPs was  . The negative ZP of the fabricated SNPs is due to the OH group of the capping agent (CS). The current result is inconsistent with that reported in the literature, where the ZP of the measured sample solution was above
. The negative ZP of the fabricated SNPs is due to the OH group of the capping agent (CS). The current result is inconsistent with that reported in the literature, where the ZP of the measured sample solution was above  or below
or below  , which were considered stable [47, 50, 51].
, which were considered stable [47, 50, 51].
3.4. Catalytic activity studies
To investigate the catalytic reduction of 4-NP to 4-AP in the presence of excessive  , the reaction progress was monitored by a UV-vis spectrophotometer. 4-NP (2 ml;
, the reaction progress was monitored by a UV-vis spectrophotometer. 4-NP (2 ml;  ) with a yellow colour showed absorption peaks at
) with a yellow colour showed absorption peaks at  and
and  [figure 8(a)] due to
[figure 8(a)] due to  (from the ring of phenol) and
(from the ring of phenol) and  (from a lone pair of electron in the oxygen and nitrogen atoms), respectively [52]. Hence, after the addition of
(from a lone pair of electron in the oxygen and nitrogen atoms), respectively [52]. Hence, after the addition of  , the colour of the reaction mixture changed to yellow green and the absorption peak was shifted to 400 nm due to the formation of 4-NP ions as shown in figure 8(a). This reaction could stay unchanged for several hours due to the presence of −NO2 group of 4-NP aromatic compounds, which is inert to the reduction of
, the colour of the reaction mixture changed to yellow green and the absorption peak was shifted to 400 nm due to the formation of 4-NP ions as shown in figure 8(a). This reaction could stay unchanged for several hours due to the presence of −NO2 group of 4-NP aromatic compounds, which is inert to the reduction of  .
.
Figure 8. UV-vis absorption spectra (ii) before and (i) after the addition of  and effect of silver/starch nanocomposites using 1 mmol l−1
and effect of silver/starch nanocomposites using 1 mmol l−1  in the presence of starch (10 g l−1) for 15 min at
in the presence of starch (10 g l−1) for 15 min at  ; on the degradation of 4-NP. UV-vis absorption spectra of the degradation of 4-NP by
; on the degradation of 4-NP. UV-vis absorption spectra of the degradation of 4-NP by  in the presence of silver/starch nanocomposite that was synthesised at (b) pH 7, (c) pH 9, and (d) pH 11.
in the presence of silver/starch nanocomposite that was synthesised at (b) pH 7, (c) pH 9, and (d) pH 11.
Download figure:
Standard image High-resolution imageMoreover, the mutually repelling negative ions 4-NP and  suggest that this reaction cannot proceed at all [5]. This is a probe catalytic reaction model, which is thermodynamically preferred (
suggest that this reaction cannot proceed at all [5]. This is a probe catalytic reaction model, which is thermodynamically preferred ( of 4-NP/4-AP is
of 4-NP/4-AP is  ). The large potential barrier between the donor
). The large potential barrier between the donor  and acceptor [4-NP] molecules decreases the feasibility of this reaction. To overcome this kinetic potential barrier, the addition of metallic NPs simplifies the catalytic reduction of 4-NP to 4-AP. The removal of 4-NP, which is one of the most carcinogenic, toxic compounds, is the primary target. The intermediate product 4-AP has medical and industrial applications such as paracetamol and dye production. The addition of 0.3 ml of fabricated SNPs, at optimized synthesis conditions (i.e. 1 mmol l−1
and acceptor [4-NP] molecules decreases the feasibility of this reaction. To overcome this kinetic potential barrier, the addition of metallic NPs simplifies the catalytic reduction of 4-NP to 4-AP. The removal of 4-NP, which is one of the most carcinogenic, toxic compounds, is the primary target. The intermediate product 4-AP has medical and industrial applications such as paracetamol and dye production. The addition of 0.3 ml of fabricated SNPs, at optimized synthesis conditions (i.e. 1 mmol l−1  in the presence of CS (10 g l−1) for 15 min at
in the presence of CS (10 g l−1) for 15 min at  ), decreased the absorption characteristic peak at 400 nm, fading with time depending on the size of the nanocatalyst at different pH (7, 9 and 11), as shown in figures 8(b)–(d); a new growing peak was assigned at
), decreased the absorption characteristic peak at 400 nm, fading with time depending on the size of the nanocatalyst at different pH (7, 9 and 11), as shown in figures 8(b)–(d); a new growing peak was assigned at  . The rate of catalytic reduction was accelerated by elevating the reaction medium pH. The reaction time was approximately 16 min for silver/starch nanocomposite that was synthesised at neutral and alkaline medium with pH 7, whereas it was 9 min and 8 min for the preparation medium of the reaction at pH 9 and 11, respectively. This may be due to the small size of the synthesised SNPs, confirmed by HR-TEM images, representing a higher surface-volume ratio that eases the catalytic reduction by facilitating the transfer of electrons from the donor ion to the acceptor ion as shown in figure 9 [53]. In order to evaluate quantitatively, the catalytic activities of the fabricated silver nanocatalyst, the following pseudo first-order rate kinetic reaction was used as the model reaction:
. The rate of catalytic reduction was accelerated by elevating the reaction medium pH. The reaction time was approximately 16 min for silver/starch nanocomposite that was synthesised at neutral and alkaline medium with pH 7, whereas it was 9 min and 8 min for the preparation medium of the reaction at pH 9 and 11, respectively. This may be due to the small size of the synthesised SNPs, confirmed by HR-TEM images, representing a higher surface-volume ratio that eases the catalytic reduction by facilitating the transfer of electrons from the donor ion to the acceptor ion as shown in figure 9 [53]. In order to evaluate quantitatively, the catalytic activities of the fabricated silver nanocatalyst, the following pseudo first-order rate kinetic reaction was used as the model reaction:

where  is the pseudo first-order rate constant,
is the pseudo first-order rate constant,  is the reaction time,
is the reaction time,  is the absorbance at a certain time and
is the absorbance at a certain time and  is the absorbance at time
is the absorbance at time  .
.
Figure 9. The proposed catalytic mechanism for the reduction of 4-NP to 4-AP catalysed by SNPs in the presence of  .
.
Download figure:
Standard image High-resolution imageThe absorbance can be estimated from the absorbance peak at 400 nm. Figure 10 shows the graphical representation of ![${\rm ln}[A]$](https://content.cld.iop.org/journals/2043-6262/9/2/025013/revision1/ansnaac4ebieqn133.gif) against the reaction time (
against the reaction time ( ), where the rate constant (
), where the rate constant ( ) can be estimated from the mentioned relationship. Hence, the rate constant for the silver/starch nanocomposite prepared at pH 7 was
) can be estimated from the mentioned relationship. Hence, the rate constant for the silver/starch nanocomposite prepared at pH 7 was  , whereas it was
, whereas it was  and
and  for SNPs synthesised at pH 9 and 11, respectively. This result confirms that the smaller the used NPs, the faster the 4-NP catalytic reduction rate (table 1).
for SNPs synthesised at pH 9 and 11, respectively. This result confirms that the smaller the used NPs, the faster the 4-NP catalytic reduction rate (table 1).
Table 1. Catalytic activity of SNPs for the reduction of 4-NP.
| Reaction medium pH | Reaction time (min) | Ka (s) | Correlation coefficient  |
|---|---|---|---|
| 7 | 16 | 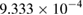 |
0.9465 |
| 9 | 9 | 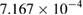 |
0.9253 |
| 11 | 8 |  |
0.9574 |
Figure 10. UV-vis absorption spectrum of the first-order linear plot of ln [A] versus reaction time.
Download figure:
Standard image High-resolution image4. Conclusion
In the present study, a simple, rapid, cost-effective and eco-friendly approach was developed to synthesise SNPs. Various reaction conditions were investigated to obtain optimum reaction conditions. The fabricated SNPs were characterised by UV-vis spectroscopy, HR-TEM, XRD, EDX, ZP and FT-IR spectroscopy. The crystalline nature of the synthesised SNPs was identified from the XRD pattern peaks and was confirmed by the selected area electron diffraction pattern. In addition, the calculated average particle size of the SNPs from the TEM histogram particle distribution was approximately  (
( ) nm for pH 11. The catalytic performance of the synthesised SNPs was investigated by reducing 4-NP to 4-AP in the presence of
) nm for pH 11. The catalytic performance of the synthesised SNPs was investigated by reducing 4-NP to 4-AP in the presence of  . The synthesised nanocatalyst at optimum reaction conditions (i.e. time = 15 min and temperature =
. The synthesised nanocatalyst at optimum reaction conditions (i.e. time = 15 min and temperature =  ) at higher pH (pH 11) exhibited potential catalytic activity compared to a lower pH (pH 7 and 9). Therefore, the synthesised silver/starch nanocomposite can be used on a large scale for various nanocatalysis applications such drug industries, wastewater removal and environmental protection.
) at higher pH (pH 11) exhibited potential catalytic activity compared to a lower pH (pH 7 and 9). Therefore, the synthesised silver/starch nanocomposite can be used on a large scale for various nanocatalysis applications such drug industries, wastewater removal and environmental protection.
Acknowledgment
The author gratefully acknowledges the help and support of Prof. Dr. Gamal Osman to complete this work.
Conflict of interest
The author declares that there is no conflict of interests regarding the publication of this manuscript.