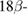Abstract
Targeting the tumor tissues in oncotherapeutics is attracting more attention worldwide for the past three decades. The exigent need to reduce the side effects of the drugs presses the need for the advancements in the targeting therapies and better alternative to the conventional chemotherapies. The effective bio compatible bioactive compounds that are appropriate substitutes to the conventional anti-cancer drugs face the difficulty in being transported across the cell membrane as they are hydrophilic. In this article the nano vesicular drug delivery system, one of the well-received approaches for targeted drug delivery is reviewed, more specifically the phytosomes. Phytosomes are the unique class of vesicular drug delivery systems that carry the plant derived bio active compounds across the cell membrane. Phytosomes are micelles capable of encapsulating the plant extracts in the core and conjugating the targeting proteins on the outer surface. Vesicular drug delivery systems in common are passive targeting drug carriers by evading the immune system. But in the case of tumor therapy, due to enhanced permeation and retention effect the phytosomes that are more than 40 kDa and a nano-metric size range of 100–1200 nm target the tumor cells actively. Passive targeting increases the bioavailability of the drugs and the active targeting specifically delivers the drugs in the site of action are coupled in phytosomes to deliver the bioactive compounds. In this review, the synthesis, properties and drug encapsulation and delivery mechanisms of the phytosomes are discussed.

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The conventional systemic drug delivery methods are prone to difficulties such as off-target effects, adverse toxic effects, and short circulation time leading to undesired side effects [1–3]. Targeted therapy hence garnered the attention of the research interest widely to address the aforementioned conundrum. A clear analysis and scientific understanding of properties of the tumor, vascular modifications, and metastatic pathways have helped to develop the targeted drug delivery system [4].
Vesicular drug delivery system (VDDS) that includes liposomes, niosomes, ethosomes, aquasomes, and phytosomes is reported to be the ideal approach satisfying all the requirements for a targeting effective drug carrier [5–9]. VDDS are micelles made up of an aqueous core and generally lipid bilayer outer shell. The inner aqueous core encapsulates the hydrophilic drugs, while lipid bilayer entraps the lipophilic moieties. This dual ability makes the VDDS, an excellent vehicle for delivery of both types of drugs [10].
Phytosomes among the VDDS are unique for they have bio active compounds as their core formulation along with phospholipid. They are the micelles formulated by the conjugation of herbal extract or aromatic active phytoconstituents such as flavonoids, terpenoids, and tannins to phospholipids in a non-polar solvent [11]. The lipid bilayer of the phytosomes helps 'contact-facilitated drug delivery (CFDD)' in which there is a lipid-lipid interaction between the carrier and the cell membrane leading to diffusion of bioactive compounds into the cell. The VDDS are usually passive targeting carriers evading the immune system and utilizing the unique deformed nature of the tumor tissues. But the phytosomes with appropriate activation on the surface with the targeting moieties serve as active targeting carriers. The phytosome micelles reduced to nanoscale are more effective due to their invasive ability. Thus the phytosomes are ideal carriers to transport the herbal bio active compounds to the target site.
In this review, the preparation of targeting phytosomes, their entrapment and drug release are discussed.
2. Phytosomes
The phytosome technology was first developed by Indena S.p.A, Italy. The polyphenols active pharmaceutical ingredients are sparsely soluble in both water and lipids. This phenomenon makes the polyphenolic APIs difficult to be formulated into commercial medicines. In 1991, Bombardelli and Spelta have formulated a new drug delivery system and named it phytosomes. Phytosomes are micelles formed by the interaction of phospholipids and water. The interaction is intensified with the addition of polyphenolic plant extracts. The polar functional groups of the lipophilic compounds conjugate through hydrogen bonds and polar interaction with the charged phosphate head of phospholipids, forming a micelle arrangement [12]. The polyphenolic compounds are encapsulated into these micelles. The majorly used phospholipid for phytosome synthesis is phosphatidylcholine that has a bifunctional molecule with hydrophilic choline head and hydrophobic phosphatidyl tail group (figure 1). The head choline group binds with the compound, while the tail phosphatidyl portion envelopes the hydrophobic drugs.
Figure 1. Structural elucidation of phosphatidyl choline.
Download figure:
Standard image High-resolution imagePhytosomes are different from liposomes in which the bio active compound is dissolved in a medium and is contained in the core cavity or in between the layers of the shell membrane. In phytosomes, the bio active compound forms an integral part of the micelle where the molecules are anchored to the polar heads of the phospholipids through chemical bonds. Liposomes are used to deliver only the water-soluble compounds while the phytosomes deliver both water soluble and lipid soluble compounds and is preferred in skin treatment over the liposomes. Indena S.p.A, a pharmaceutical company from Italy is producing ranges of pharmaceutical products with phytosome as the base for various ailments. The products are listed in table 1.
Table 1. Various products based on phytosomes by the pharmaceutical industry, Indena S.p.A, Italy.
| Trade name | Phytochemical | Indication | Reference |
|---|---|---|---|
| glycyrrhetinic acid phytosome® | glycyrrhetinic acid from licorice rhizome | Soothing | [13] |
| Boswellia phytosome® | Boswellic acids from Boswellia serrata's resins | Healthy inflammatory response, soothing, lenitive | [14] |
| Centella phytosome® | Triterpenes from Centella asiatica leaf | Collagen restructurant, anti-wrinkle agent | [15] |
| Crataegus phytosome® | Vitexin-2''-O-rhamnoside from Hawthorn flower | Antioxidant | [16] |
| Escin sitosterol phytosome® | Escin sitosterol from horse chestnut fruit | Capillarotropic | [17] |
| Ginkgoselect® phytosome® | |||
| Ginselect® phytosome® | Ginsenosides from Panax ginseng rhizome | Adaptogen, tonic, skin elasticity improver | [18] |
| Ginkgo biloba terpenes phytosome® | Ginkgolides and bilobalide from Ginkgo biloba leaf | Soothing | [19] |
| Ginkgo biloba dimeric flavonoids phytosome® | Dimeric flavonoids from Ginkgo biloba leaf | Lipolytic, vasokinetic | [20] |
| Greenselect® phytosome® | Polyphenols from Camellia sinensis leaf | Weight loss agent, antioxidant | [21] |
| Leucoselect® phytosome® | Polyphenols from grape seed | Antioxidant, capillarotropic | [22] |
| Meriva® | Curcuminoids from turmeric rhizome | Joint health, healthy inflammatory response | [23] |
| PA2 phytosome® | Proanthocyanidin A2 from horse chestnut bark | Anti-wrinkles, UV protectant | [24] |
| Resveratrol phytosome® | Resveratrol from Polygonum cuspidatum's rizhome | Antioxidant | [25] |
| Sericoside phytosome® | Sericoside from Terminalia sericea bark root | Anti-wrinkles | [26] |
| Siliphos® | Silybin from milk thistle seed | Healthy liver, retinoic acid-like compound | [27] |
| Silymarin phytosome® | Silymarin from milk thistle seed | Healthy liver, antioxidant, UV protectant | [28] |
| Virtiva® | Ginkgo flavone glucosides, ginkgolides, bilobalide from Ginkgo biloba leaf | Vasokinetic | [29] |
| Visnadex® | Visnadin from Amni visnaga umbel | Vasokinetic | [30] |
2.1. Phytosome preparation
Phytosomes are prepared by de-hydration and re-hydration technique as depicted in figure 2. The bio active compound along with the phospholipid is dissolved in organic solvent. The organic solvent is then eliminated completely along with the aqueous content under a reduced temperature and pressure using a rotary vacuum evaporator. A thin layer containing a conjugated complex of phospholipid and bioactive compound would be formed in the round bottom flask. The thin layer is countered with hexane to remove the solvents completely. Then the thin layer is re hydrated with water to form micelles. The phospholipid thin layer upon exposure with the water forms micelles that are then probe sonicated to achieve desired micelle size [9, 31–33].
Figure 2. Dehydration-rehydration preparatory process of the phytosomes.
Download figure:
Standard image High-resolution image2.2. Release from phytosomes
Phytosomes are advantageous while delivering lipophilic drug components. Lipophilic compounds tend to form clusters in the intestine due to hydrolytic digestion. This cluster formation would hinder the sustained and controlled release of the drug into the circulatory system. This phenomenon is remedied in phytosomal delivery where the phosphatidyl choline forms a monolayer in the digestive track, prevents the cluster formation, and enhances the diffusion of lipophilic drugs through the brush border of the small intestine [34]. The entrapment efficiency of the phytosome has been studied by various research teams and reported to be 86%–98% and the higher efficiency could be due to the bond between the phytochemical and the polar head of the charged phospholipid encouraging the conjugation of phytochemicals with the polar heads both outer and inner walls of the vessel. The release has been reported to be time-dependent and diffusion controlled. The total amount of release of drugs was measured to be 80%–85% from the phytosome complex. The rate of release is slower than liposome due to the association of the drug with the phosphatidyl head [34–37]. The time-dependent release of the drug from phytosome can be attributed to the stability of the phytosomal shell.
2.3. Targeting phytosomes
2.3.1. Passive targeting.
The delivery of the drugs to the site of target through convection is passive targeting [38]. The evasion of drugs from the immune response by stealth mechanism to increase the bioavailability of the drugs is another class of passive targeting [39]. Enhanced permeation and retention (EPR) effect and compromising the reticuloendothelial system (RES) are the two passive targeting approaches ideal for tumor therapy [40].
2.3.1.1. Enhanced EPR effect.
The pharmacokinetic profile of different proteins was studied (molecular weights ranging from 12 to 150 kDa correlated with the rate of tumor uptake) by Matsumura and Maeda in 1986 and evaluated that the long half-life in the blood circulation is a prerequisite for an enhanced tumor uptake of the protein [41]. They also observed that there was no significant difference in accumulation between albumin (MW 66.5 kDa) and an immunoglobulin (MW 150 kDa) in the tumor. Based on the tumor uptake of molecules, tumor blood flow, transport of molecules in the interstitium and retention of the molecules, they have proposed a principle mechanism termed EPR of macromolecules in relation to passive tumor targeting [42]. The endothelial barriers in the healthy tissue blood vessels permeate only small molecules of size less than 100 nm. But the pore size of tumor micro vessels varies from 100 to 1200 nm in diameter and these leaky defective blood vessels make its vasculature permeable for macromolecules [43, 44]. Macromolecules that are larger than 2 nm and smaller than 10 nm as carriers for the development of macromolecular prodrugs extravasate into tumor tissue but not into normal tissue.
Apart from enhanced permeability, the clearance rate from the tumor is reduced when the molecular weight exceeds 40 kDa [45]. The smaller molecules are rapidly cleared from the tumor interstitium, while the larger molecules are retained. This enhanced retention of macromolecules in tumor tissue is primarily caused by a lack of lymphatic drainage due to an impaired or absent lymphatic system. Hence, it is the combination of both an EPR ensuring the targeting, accumulation, and bioavailability of macromolecules in solid tumors [46].
2.3.1.2. Evading RES.
Drugs and drug carriers usually undergo first pass mechanism where they enter liver from duodenum, excipients removed, get activated, and finally exposed to the plasma of the circulating blood. This first pass mechanism checks the intrusion of harmful toxic agents from entering the circulatory system. This is the RES where the immune response is triggered against particles that are larger than 100 nm. Drugs or drug carriers with the hydrophobic surface are preferentially taken up by liver, spleen, and lungs [47]. Drug carriers that have hydrophilic surface had shown less than 1% uptake by spleen and liver [44]. Liposomes, however, having the hydrophilic surface are easily cleared from the system by a process called opsonization in the liver. The opsonin protein present in the blood ligates itself with the foreign particles (drugs) and makes them recognizable to phagocytes in the liver. The phagocytes confiscate these particles and remove them from circulation. But the phytosome has been proved to evade the RES as they have the hydrophilic surface associated with drug molecules making them nonspecific for the biding with opsonin or plasma proteins in the blood and their micelle size range lies in 30–100 nm [48, 49].
2.3.2. Active targeting.
For active targeting, it is required to understand the pathway of each cancer so as to treat the tumors in the most specific approach. Some of the pathways and the possible targeting modes are reviewed below.
2.3.2.1. The mTOR signaling pathway.
The mammalian target of rapamycin (mTOR) is a 389 kDa kinase enzyme that has two protein sub complexes, mTOR complex 1 (negative feedback) and 2 (positive feedback). The mTOR protein is also termed as FKBP12-rapamycin-associated protein. The PI3K/Akt/mTOR pathway regulates the cell survival, quiescence, senescence, macrophage polarization, and proliferation depending on the availability of nutrients [50]. The mTOR signaling may be abnormally or inappropriately activated irrespective of nutrient status extracellularly due to mutation. Mutations involving mTOR pathway are well recognized in breast cancer, ovary, colon and renal cell carcinomas [4]. Growth factors and nutrients upregulate this pathway while tumor suppressor gene phosphatase and tensin homolog (PTEN) down-regulates the pathway [51]. Deficiency of PTEN causes disproportionate activation of the mTOR pathway and this has been observed in renal cell carcinoma and breast cancer. Targeting the mTOR pathway has shown a promising clinical benefit in cancers in the breast, kidney, neuro endocrine origin tumors, glioblastoma [52–54]. Currently, two mTOR inhibitors are approved by the FDA and European Medical association: Temsirolimus and Everolimus. Everolimus is 40-O-(2-hydroxyethyl) derivative of sirolimus oral mTOR inhibitor approved for breast cancer. It inhibits mTOR complex 1 specifically and does not make any impact on mTOR complex 2 [55].
Temsirolimus and everolimus are mTOR inhibitors targeting the mTOR pathway by inhibiting tumor angiogenesis by reducing the synthesis of vascular endothelial growth factors (VEGF) for advanced renal cell carcinoma [56]. Conjugating the everolimus along with the phytosome can target the breast cancer cells as they have disproportionate mTOR negative feedback pathway while conjugating the temsirolimus will target the renal cancer pathway and act only on the tumors.
2.3.2.2. The VEGF receptor pathway.
Vascular endothelial growth factor (VEGF) and VEGF receptor pathway are the key regulators of the angiogenesis in the tumor. VEGF, binding to its receptor VEGFR, could fuel tumor growth. Growth, migration, and differentiation of endothelial cells are upregulated by a cascade of signals from activated VEGF/VEGF-receptor pathway. The role of VEGF in promoting tumor angiogenesis and the occurrence of human cancers has led to the rational design and development of agents that selectively target this pathway [57]. Judah Folkman hypothesized the idea that angiogenesis can be targeted in cancer by nearly four decades ago [58]. Extensive research on angiogenesis revealed that the ligand vascular endothelial growth factor (VEGF) and VEGF receptors can be targeted. There are five different ligands and three types of receptors in VEGF family among which VEGFA and VEGFR 2 are prominent in tumorigenesis. The anti-angiogenic molecules in the form of either VEGF ligand inhibitors, decoy receptors or kinase inhibitors of the intracellular domain of VEGF receptors are the targeting moieties that can be used to target the anti-angiogenesis therapy [59]. The monoclonal antibodies along with the angiogenesis inhibitors like bevacizumab and ranibizumab attached on the surface of the phytosome would target the pro-angiogenic members of the VEGF family [60].
2.3.2.3. Peptide sequence targeting.
This approach depends on interactions with proteins and enzymes found specifically on the surface of cancerous cells and not the surface of healthy cells. Most linkers are usually peptidase-cleavable or acid labile [4]. A research on conjugation of doxorubicin with poly(ethylene glycol) linker with enzymatically cleavable peptide sequences (alanyl-valine, alanyl-proline, and glycyl-proline) had expressed a strong specificity to get cleaved only at tumor cells releasing the drug, doxorubicin [61]. Activating the surface of the phytosome immediately after the rehydration process with the tumor specific enzymatically cleavable peptide sequences can be best targeting drug carrier.
3. Conclusion
The phytosome technology is booming in the pharmaceutical industry for its ability to deliver drugs targeted both passive and active. The preparatory method of phytosome is simpler than most drug carrier preparations. A deeper study on the mechanism of any disease is needed to formulate the targeting strategy and moieties. Phytosome hence can be used to deliver drugs for malfunctions other than tumor as well. The sources for the preparation of phytosomes are sparsely studied as the research is still in shadow era. The very fact that the plant materials required to formulate the phytosome themselves act as active pharmaceutical ingredient makes this approach more viable and novel. There is a humongous scope for the phytosome technology development in the future.
Acknowledgments
We are thankful to the management of Bannari Amman Institute of Technology for providing us the facility to conduct our research and this review is a part of the research being carried out on phytosomal delivery of drugs.