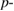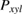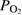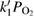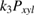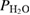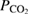Abstract
In this investigation, the thin film of  degussa P25 was obtained by dip coating method and calcined at 450 °C for 2 h (P25-450-2) and used as photocatalyst for gas-phase photooxidation of
degussa P25 was obtained by dip coating method and calcined at 450 °C for 2 h (P25-450-2) and used as photocatalyst for gas-phase photooxidation of  xylene. The physico-chemical properties of calcined P25-450-2 powder was studied by the methods of BET adsorption, XRD, FTIR, UV–vis, Raman spectroscopies, SEM, TEM, carbon dioxide temperature-programmed desorption
xylene. The physico-chemical properties of calcined P25-450-2 powder was studied by the methods of BET adsorption, XRD, FTIR, UV–vis, Raman spectroscopies, SEM, TEM, carbon dioxide temperature-programmed desorption  . The thickness of the film was determined on the Alpha Step IQ KLA—Ctencor equipment and the point of zero charge (PZC) of the sample was determined by salt addition method. P25-450-2, having a band gap of 3.155 eV, is advisable to use UV lamps in photocatalytic reactions. The kinetics of gas-phase photooxidation of
. The thickness of the film was determined on the Alpha Step IQ KLA—Ctencor equipment and the point of zero charge (PZC) of the sample was determined by salt addition method. P25-450-2, having a band gap of 3.155 eV, is advisable to use UV lamps in photocatalytic reactions. The kinetics of gas-phase photooxidation of  xylene reaction on the thin films of P25-450-2 under UV illumination was studied using a gradientless flow circulating system at atmospheric pressure and 40 °C. The obtained results showed that the kinetics of the given reaction should be written by fractional equations describing the dependence of the reaction rate on the concentration of adsorbed molecules of
xylene reaction on the thin films of P25-450-2 under UV illumination was studied using a gradientless flow circulating system at atmospheric pressure and 40 °C. The obtained results showed that the kinetics of the given reaction should be written by fractional equations describing the dependence of the reaction rate on the concentration of adsorbed molecules of  xylene and oxygen, dissociative adsorbed water vapor, and also on the total intensity of light. The reaction was proposed to follow the Langmuir-Hinshelwood mechanism.
xylene and oxygen, dissociative adsorbed water vapor, and also on the total intensity of light. The reaction was proposed to follow the Langmuir-Hinshelwood mechanism.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Photocatalytic oxidation (PCO) is increasingly playing an important role in environmental science. PCO is considered to be a heterogeneous catalytic reaction with individuality due to the it's activation by light, and studies in this field focus on: (i) find new semiconductor catalysts working better in a more diverse light region; (ii) study to clarify the behavioral characteristics of the reaction.
Until now,  have been evaluated as the most effective catalysts in ultraviolet light [1, 2]. Studies [1–3] showed that the photocatalytic activity of
have been evaluated as the most effective catalysts in ultraviolet light [1, 2]. Studies [1–3] showed that the photocatalytic activity of  was highest at an appropriate anatase/rutile ratio. This is because the energy of valence band of the anatase and rutile phases is approximately the same, but the energy of conduction band of anatase is higher than that of rutile phase 0.3 eV. Thus, the electrons from the conduction band of anatase phase can jump to the one of rutile that limiting the rate of recombining electrons and holes. According to Bickley et al [4] the anatase/rutile structure of
was highest at an appropriate anatase/rutile ratio. This is because the energy of valence band of the anatase and rutile phases is approximately the same, but the energy of conduction band of anatase is higher than that of rutile phase 0.3 eV. Thus, the electrons from the conduction band of anatase phase can jump to the one of rutile that limiting the rate of recombining electrons and holes. According to Bickley et al [4] the anatase/rutile structure of  P25 promotes charge-pair separation and inhibits recombination. Ohno et al [5] found that
P25 promotes charge-pair separation and inhibits recombination. Ohno et al [5] found that  P25 with 75% anatase phase showed higher photocatalytic activity than 100% anatase catalysts (ST01, ST21) and 100% rutile free
P25 with 75% anatase phase showed higher photocatalytic activity than 100% anatase catalysts (ST01, ST21) and 100% rutile free  (PT101). So far,
(PT101). So far,  P25 is considered as the most effective bare
P25 is considered as the most effective bare  catalyst for the photocatalytic reaction under UV light.
catalyst for the photocatalytic reaction under UV light.
In general, the analysis of the data in the references shows that for the gas-phase PCO there is a small amount of kinetic studies [6], and although there are various proposed kinetic models, most authors agreed that the photooxidation process is a heterogeneous reaction occurring on surface of the catalysts between the adsorbed substance with oxygen in adsorption state (Langmuir-Hinshelwood model), from volume phase (Eley-Rideal model), or lattice O of  (Mars-Van Krevelen model). Kinetic models of the propane photo-oxidation over a vanadium-doped
(Mars-Van Krevelen model). Kinetic models of the propane photo-oxidation over a vanadium-doped  catalyst were studied in a fixed-bed reactor, using 4 g of catalyst, under ultraviolet light irradiation are summarized in [7]. These models include three main mechanisms: Langmuir-Hinshelwood (LH), Eley-Rideal (ER) and Mars-Van Krevelen (MVK). According to the LH mechanism, the surface reaction between adsorbed-competing propane and oxygen was considered as the rate-determining step and common kinetic equation was accepted as following:
catalyst were studied in a fixed-bed reactor, using 4 g of catalyst, under ultraviolet light irradiation are summarized in [7]. These models include three main mechanisms: Langmuir-Hinshelwood (LH), Eley-Rideal (ER) and Mars-Van Krevelen (MVK). According to the LH mechanism, the surface reaction between adsorbed-competing propane and oxygen was considered as the rate-determining step and common kinetic equation was accepted as following:
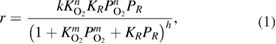
where  is reaction rate constant;
is reaction rate constant;  and
and  are adsorption equilibrium constant of oxygen and organic substrate, respectively, and
are adsorption equilibrium constant of oxygen and organic substrate, respectively, and  is their partial pressure;
is their partial pressure;  are the orders of corresponding reactant, and
are the orders of corresponding reactant, and  is surface coverage.
is surface coverage.
According to the Eley-Rideal mechanism, chemically adsorbed oxygen reacts with propane from the gas phase and the reaction kinetics is described by the following common equation:
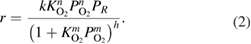
The Mars–Van Krevelen (MVK) mechanism has a reduction stage, in which propane reacts with single or two oxidized surface sites and a stage of re-oxidation of the reducing centers on the surface by oxygen from gas phase and the kinetic equation receives the following common form:
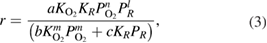
where  are the kinetic constants; and
are the kinetic constants; and  are the order of corresponding reactants.
are the order of corresponding reactants.
For most heterogeneous catalytic reactions the Langmuir model is widely accepted [8–11]. Many authors showed that the Langmuir-Hinshelwood model is suitable for the reactions of gas-phase photocatalytic oxidation of organic compounds [12], acetone [13], methyl tert-butyl ether (MTBE) [14, 15] and the kinetic equation has the form
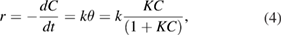
where  is adsorption equilibrium constant of the reactant, and
is adsorption equilibrium constant of the reactant, and  is concentration of the reactant at time of observation. In paper [16], the photocatalytic activity of a
is concentration of the reactant at time of observation. In paper [16], the photocatalytic activity of a  thin film in degradation of gaseous methanol and toluene under the UV light irradiation in accordance with the LH kinetic model.
thin film in degradation of gaseous methanol and toluene under the UV light irradiation in accordance with the LH kinetic model.
For the effect of photon flux, it was found that the reaction rate  depends on the photon flux of UV light
depends on the photon flux of UV light  in a power law [17]
in a power law [17]

where  is the reaction rate independent of the photon flux, and
is the reaction rate independent of the photon flux, and  is the order of photon flux.
is the order of photon flux.
According to authors [17–19] the order of photon flux  in equation (5) depends on the light intensity and the wavelength light, the first-order was observed when the illumination intensity is noticeably lower than
in equation (5) depends on the light intensity and the wavelength light, the first-order was observed when the illumination intensity is noticeably lower than  , and the half-order at the photon flux above
, and the half-order at the photon flux above  . In the photo-oxidation of methanol and toluene it has been found that depending on wavelength of the UV light the order of
. In the photo-oxidation of methanol and toluene it has been found that depending on wavelength of the UV light the order of  is 0.42 and 0.57 respectively under the irradiation of germicidal lamps
is 0.42 and 0.57 respectively under the irradiation of germicidal lamps  or is 0.89 and 0.95 respectively under the irradiation of black light lamps
or is 0.89 and 0.95 respectively under the irradiation of black light lamps  [17]. However, Jaime et al [13] reported that the dependence of the reaction rate of the photocatalytic process on the photon intensity was not detected.
[17]. However, Jaime et al [13] reported that the dependence of the reaction rate of the photocatalytic process on the photon intensity was not detected.
The aim of this work is to evaluate the photocatalytic activity and stability of thin film  degussa P25, calcined at 450 °C for 2 h, for gas-phase photooxidation of
degussa P25, calcined at 450 °C for 2 h, for gas-phase photooxidation of  xylene under UV illumination and to clarify the kinetics of the reaction.
xylene under UV illumination and to clarify the kinetics of the reaction.
2. Experimental
The  P25 film on pyrex glass tube was obtained by submerged coating from a suspension of
P25 film on pyrex glass tube was obtained by submerged coating from a suspension of  P25 according to the procedure described in detail in our previous publication [20]. The catalyst tubes were calcined at 450 °C for 2 h and catalyst is denoted by P25-450-2. Supports for the active phase of the catalysts were closed pyrex glass tubes with 19 mm in diameter, 270 mm in length and treated with aqueous hydrofluoric acid solution.
P25 according to the procedure described in detail in our previous publication [20]. The catalyst tubes were calcined at 450 °C for 2 h and catalyst is denoted by P25-450-2. Supports for the active phase of the catalysts were closed pyrex glass tubes with 19 mm in diameter, 270 mm in length and treated with aqueous hydrofluoric acid solution.
The physico-chemical properties of P25-450-2 powder catalysts were characterized by methods of Brunauer-Emmett-Teller (BET) adsorption using BET Nova 2200E equipment; x-ray diffraction (XRD) on a D8 Advance Bruker powder diffractometer; Fourier-transform infrared (FTIR) spectroscopy using Tensor 27-Bruker; scanning electron microscope (SEM) on FE-SEM JEOL 7401 and transmission electron microscopy (TEM) on JEM 1400 (Jeol USA); Raman by using Labram-HR -Horiba Jobin Yvon, and UV–vis on LUV-300 single beam spectrophotometer (USA). The point of zero charge (PZC) of obtained P25-450-2 catalyst was determined by salt addition method [21]. The basicity of catalysts was investigated by the carbon dioxide temperature-programmed desorption  according to the following procedure. After activation in air at 450 °C for 2 h the sample was adsorbed
according to the following procedure. After activation in air at 450 °C for 2 h the sample was adsorbed  at room temperature for 1 h. Then, the sample was blown with inert gas
at room temperature for 1 h. Then, the sample was blown with inert gas  at 50 °C for 1 h and heated from 50 °C to 700 °C at a rate of 10 °C min−1. The desorption volume of
at 50 °C for 1 h and heated from 50 °C to 700 °C at a rate of 10 °C min−1. The desorption volume of  was monitored by using a gas chromatograph Gowmac 69-350 with a thermal conductivity detector. The thickness of the film is determined on the Alpha Step IQ KLA—Ctenco equipment.
was monitored by using a gas chromatograph Gowmac 69-350 with a thermal conductivity detector. The thickness of the film is determined on the Alpha Step IQ KLA—Ctenco equipment.
The kinetics of the gas-phase photooxidation of  xylene was carried out in a gradientless flow circulating system at atmospheric pressure and 40 °C. The reaction was carried out using 15 mg of thin film catalyst having illuminated area of 65 cm2. Three UV lamps (
xylene was carried out in a gradientless flow circulating system at atmospheric pressure and 40 °C. The reaction was carried out using 15 mg of thin film catalyst having illuminated area of 65 cm2. Three UV lamps ( with the power of 8 W/lamp) were used as the irradiation sources for photoreaction. Prior to the reaction, the catalysts were saturated with
with the power of 8 W/lamp) were used as the irradiation sources for photoreaction. Prior to the reaction, the catalysts were saturated with  xylene in the dark. To analyze the hydrocarbons composition of reaction mixture the gas-chromatograph Agilent 6890 Plus GC, FID detector, and capillary column DB-624 (30 m length; 0.32 mm outer diameter;
xylene in the dark. To analyze the hydrocarbons composition of reaction mixture the gas-chromatograph Agilent 6890 Plus GC, FID detector, and capillary column DB-624 (30 m length; 0.32 mm outer diameter;  inter diameter) were used.
inter diameter) were used.  measurement was done on GC-MS 6890N/MSD5973 with MS-5973N detector using capillary column HP-Plot Q.
measurement was done on GC-MS 6890N/MSD5973 with MS-5973N detector using capillary column HP-Plot Q.
3. Results and discussion
3.1. The properties and the activity of photocatalyst
The effect of calcination temperature on the physico-chemical properties of  P25 catalyst was investigated in [20]. From the results it follows that calcination has led to changes in physico-chemical properties of the samples that affect the activity of the catalysts. The sample calcined at 450 °C was characterized by biggest specific surface area, as a result, it exhibited relatively high photocatalytic activity in the illumination of ultraviolet light. Inheriting the results of the publication [20] and in order to increase the economics of catalytic membrane preparation, in this study, the
P25 catalyst was investigated in [20]. From the results it follows that calcination has led to changes in physico-chemical properties of the samples that affect the activity of the catalysts. The sample calcined at 450 °C was characterized by biggest specific surface area, as a result, it exhibited relatively high photocatalytic activity in the illumination of ultraviolet light. Inheriting the results of the publication [20] and in order to increase the economics of catalytic membrane preparation, in this study, the  P25 film after dipping, drying, was heated at 450 °C for a shorter time, of 2 h instead of 4 h as indicated in [20].
P25 film after dipping, drying, was heated at 450 °C for a shorter time, of 2 h instead of 4 h as indicated in [20].
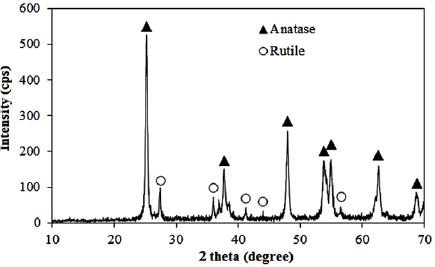
XRD pattern of P25-450-2 sample (figure 1) exhibits diffraction peaks of the anatase ( ,
,  ) and rutile phase
) and rutile phase  [22]. The acuteness of peaks in XRD pattern demonstrates high crystallinity of sample. According to the XRD patterns of the catalyst, the ratio of anatase to rutile in obtained catalyst was found at 80.9/19.1. This phase ratio of P25-450-2 sample causes the synergetic effect (40% ⩽ [anatase] ⩽ 80%) [23]. Hence, it is predicted high photoactivity to degrade organic pollutants. The average size of the P25-450-2 crystallites was estimated approximately 21 nm by using Scherrer formula at
[22]. The acuteness of peaks in XRD pattern demonstrates high crystallinity of sample. According to the XRD patterns of the catalyst, the ratio of anatase to rutile in obtained catalyst was found at 80.9/19.1. This phase ratio of P25-450-2 sample causes the synergetic effect (40% ⩽ [anatase] ⩽ 80%) [23]. Hence, it is predicted high photoactivity to degrade organic pollutants. The average size of the P25-450-2 crystallites was estimated approximately 21 nm by using Scherrer formula at  [24].
[24].
Figure 1. XRD pattern of P25-450-2 sample.
Download figure:
Standard image High-resolution imageOn the Raman spectrum of P25-450-2 sample (figure 2) the characteristic peaks of anatase  at
at  and of rutile phase at 443 and
and of rutile phase at 443 and  were observed. Furthermore, intensity of characteristic Raman peak for rutile is much lower than that for anatase. This is accordant with dominance of anatase phase as calculating phase content from the XRD result.
were observed. Furthermore, intensity of characteristic Raman peak for rutile is much lower than that for anatase. This is accordant with dominance of anatase phase as calculating phase content from the XRD result.
Figure 2. Raman spectrum of P25-450-2 sample.
Download figure:
Standard image High-resolution imageAccording to isothermal adsorption results, the BET specific surface area of P25-450-2 sample is  approximately (table 1). It is close to the results of the sample P25-450-4 in investigation [20].
approximately (table 1). It is close to the results of the sample P25-450-4 in investigation [20].
Table 1. The physico-chemical characteristics of  samples: Values of surface specific area (SBET), pore volume (
samples: Values of surface specific area (SBET), pore volume ( ), average pore size (
), average pore size ( ), TiO2 crystal size calculated at 2θ = 25.3° (
), TiO2 crystal size calculated at 2θ = 25.3° ( ), particle size (
), particle size ( ), phase ratio anatase/rutile (A/R), point of zero charge (PZC), wavelength of light absorption (λ), and band gap energy (EG).
), phase ratio anatase/rutile (A/R), point of zero charge (PZC), wavelength of light absorption (λ), and band gap energy (EG).
| Characteristics |   |
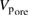 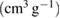 |
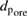 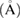 |
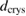 (nm) (nm) |
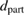 (nm) (nm) |
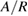 |
PZC | λ (nm) | 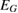 (eV) (eV) |
|---|---|---|---|---|---|---|---|---|---|
| P25-450-2 | 49.6 | 0.037 | 33.5 | 23 | 20–30 | 80.9/19.1 | 6.30 | 393 | 3.155 |
| P25-450-4 [20] | 50.0 | — | — | 33 | — | 81.6/18.4 | — | 325–430 | 3.180 |
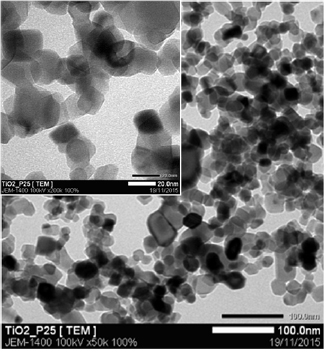
According to the SEM image (figure 3), P25-450-2 catalyst is suggested having pseudo spherical in shape. In addition, its distribution is nearly uniform. TEM image (figure 4) indicates the co-existence of both bright and dark fields, typically for the presence of anatase and rutile phase in structure of P25-450-2 catalyst [25]. From the SEM and TEM images, the average size of the primary particles estimates about 20–30 nm. This is a good agreement with the results calculated from the XRD pattern (21 nm).
Figure 3. SEM image of P25-450-2 sample.
Download figure:
Standard image High-resolution imageFigure 4. TEM image of P25-450-2 sample.
Download figure:
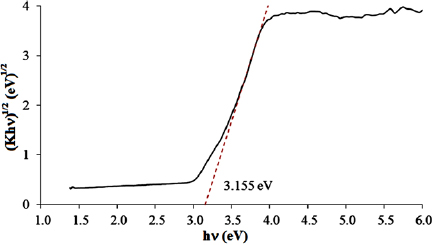
Standard image High-resolution imageAs can be seen from UV spectrum in figure 5, P25-450-2 mainly absorbs UV irradiation. Band gap energy of P25-450-2 sample based on Tauc curve (figure 6) was determined at 3.155 eV. The absorbable wavelength of the sample can be calculated by the equation  and equal 393 nm. Hence, to initiate photochemical effects in semiconductor P25-450-2, UV light was required.
and equal 393 nm. Hence, to initiate photochemical effects in semiconductor P25-450-2, UV light was required.
Figure 5. UV–vis absorbance of P25-450-2 sample.
Download figure:
Standard image High-resolution imageFigure 6. Plots of the transformed Kubelka-Munk function versus the energy of the light absorbed for P25-450-2 sample.
Download figure:
Standard image High-resolution imageAs can be seen from figure 7, strong peaks in FTIR spectrum are observed at 3419, 1632, and  . The broad peak at 3419 and the peak at
. The broad peak at 3419 and the peak at  are believed corresponding to the hydroxyl groups and surface-adsorbed water respectively, while the main peak at from 400 to
are believed corresponding to the hydroxyl groups and surface-adsorbed water respectively, while the main peak at from 400 to  is ascribed to Ti-O stretching and Ti-O-Ti bridging stretching modes. The existence of this OH-groups explains the generation of
is ascribed to Ti-O stretching and Ti-O-Ti bridging stretching modes. The existence of this OH-groups explains the generation of  on the catalyst surface.
on the catalyst surface.
Figure 7. FTIR spectrum of P25-450-2 sample.
Download figure:
Standard image High-resolution imageSo, compared to P25-450-4 sample in article [20], P25-450-2 catalyst used in this study exhibited a smaller crystal size and slightly lower band gap energy. This may due to considerably shorter calcination duration was used in this study (2 versus 4 h).
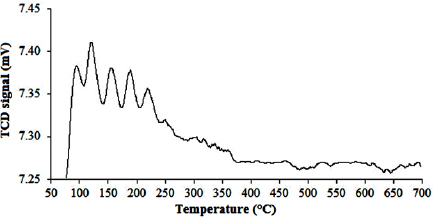
 pattern in the figure 8 exhibited the
pattern in the figure 8 exhibited the  desorption peaks in temperature range of 100 °C–350 °C. According to Wang et al [26] the
desorption peaks in temperature range of 100 °C–350 °C. According to Wang et al [26] the  desorption peaks in temperature-programmed desorption (TPD) pattern of
desorption peaks in temperature-programmed desorption (TPD) pattern of  at temperatures of 150 °C–200 °C and above 400 °C represents the weak, medium and strong basic sites, respectively. Then, the received results showed that on P25-450-2 catalyst exist the weak and medium basic sites, moreover weak basic sites dominate. Duffy [27] reported that the basicity of
at temperatures of 150 °C–200 °C and above 400 °C represents the weak, medium and strong basic sites, respectively. Then, the received results showed that on P25-450-2 catalyst exist the weak and medium basic sites, moreover weak basic sites dominate. Duffy [27] reported that the basicity of  is much greater than that of
is much greater than that of  and is nearly the same as the basicity of CaO. Bergman [28] determined the optical basicity of
and is nearly the same as the basicity of CaO. Bergman [28] determined the optical basicity of  is 0.55.
is 0.55.
Figure 8.  pattern of P25-450-2 sample.
pattern of P25-450-2 sample.
Download figure:
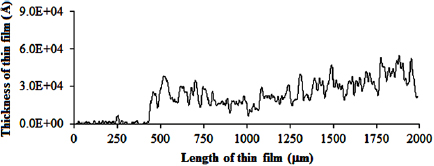
Standard image High-resolution imageFigure 9 showed a uniform distribution of  particles on the film and the thin film P25-450-2 has an average thickness of
particles on the film and the thin film P25-450-2 has an average thickness of  .
.
Figure 9. The thickness diagram of P25-450-2 film.
Download figure:
Standard image High-resolution imageFrom the mentioned above results, it follows that the  powder after calcination at
powder after calcination at  for 2 h predominantly consist of spherical shape crystals, having an anatase/rutile phase ratio of 80.9/19.1 and band gap energy
for 2 h predominantly consist of spherical shape crystals, having an anatase/rutile phase ratio of 80.9/19.1 and band gap energy  of 3.155 eV, absorbing UV light, are capable to use UV lamps in photocatalytic reactions. This catalyst exhibited relatively high activity and stability under the illumination of ultraviolet light (figure 10). This meets the requirements for studying the kinetics of the reaction. The enhancing photoactivity of P25-450-2 sample is explained by synergetic effect of the mixture anatase and rutile phase of suitable ratio [23] and the contact of two phases makes slowly electron-hole recombination [4]. In addition, the smaller size and lower band gap energy also contributed to increased activity of P25-450-2 catalyst.
of 3.155 eV, absorbing UV light, are capable to use UV lamps in photocatalytic reactions. This catalyst exhibited relatively high activity and stability under the illumination of ultraviolet light (figure 10). This meets the requirements for studying the kinetics of the reaction. The enhancing photoactivity of P25-450-2 sample is explained by synergetic effect of the mixture anatase and rutile phase of suitable ratio [23] and the contact of two phases makes slowly electron-hole recombination [4]. In addition, the smaller size and lower band gap energy also contributed to increased activity of P25-450-2 catalyst.
Figure 10. The  xylene conversion extent versus reaction time on P25-450-2 thin film at reaction temperature of 40 °C; (
xylene conversion extent versus reaction time on P25-450-2 thin film at reaction temperature of 40 °C; ( ,
,  ,
,  ,
,  , 3 UV lamps of 8 W each;
, 3 UV lamps of 8 W each;  ; total light intensity 1147 lux).
; total light intensity 1147 lux).
Download figure:
Standard image High-resolution imageThe high stability of P25-450-2 catalyst is explained by its high basicity (as shown from the  result) and the small particle size, yielding less favorable conditions to coke deposit on the catalyst surface. Furthermore, the point of zero charge of P25-450-2 catalyst was determined at
result) and the small particle size, yielding less favorable conditions to coke deposit on the catalyst surface. Furthermore, the point of zero charge of P25-450-2 catalyst was determined at  , so in the gas-phase reaction medium its surface charge is neutral, the absence of electrostatic force causes the minimum of the interplay between surface particles and contaminants. This also contributes to reducing coke deposit on the catalyst surfaces. Indeed, the by-products formed on surface of P25-450-2 catalyst during photocatalytic oxidation of
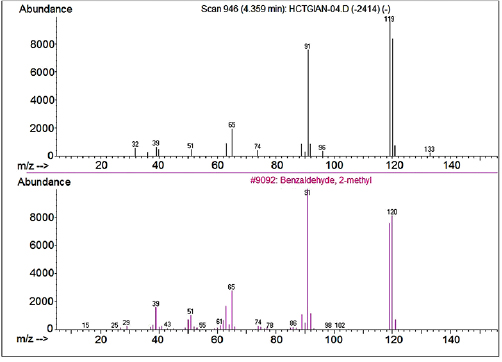
, so in the gas-phase reaction medium its surface charge is neutral, the absence of electrostatic force causes the minimum of the interplay between surface particles and contaminants. This also contributes to reducing coke deposit on the catalyst surfaces. Indeed, the by-products formed on surface of P25-450-2 catalyst during photocatalytic oxidation of  xylene were analyzed from the solution extracted in the dark from the catalyst layer in methanol after a long period of operation (several days) was estimated less than 1 mg g−1 catalyst. The results of the analysis (figures 11 and 12) showed that the compounds, depositing on the catalyst surface are products of the partial oxidation such as 2-methylbenzaldehyde and phthalic anhydride. They are oxygenate compounds with a low C: H ratio (CH0.5 for phthalic anhydride and CH for methylbenzaldehyde), easily combusted in reaction conditions, leading to the low amount of deposited products, that increased the stability of catalyst.
xylene were analyzed from the solution extracted in the dark from the catalyst layer in methanol after a long period of operation (several days) was estimated less than 1 mg g−1 catalyst. The results of the analysis (figures 11 and 12) showed that the compounds, depositing on the catalyst surface are products of the partial oxidation such as 2-methylbenzaldehyde and phthalic anhydride. They are oxygenate compounds with a low C: H ratio (CH0.5 for phthalic anhydride and CH for methylbenzaldehyde), easily combusted in reaction conditions, leading to the low amount of deposited products, that increased the stability of catalyst.
Figure 11. HPLC/MS spectrum of intermediate product extracted in methanol (the above picture) and standard spectra of phthalic anhydride (figure below).
Download figure:
Standard image High-resolution imageFigure 12. HPLC/MS spectrum of intermediate product extracted in methanol (the above picture) and standard spectra of 2-methylbenzaldehyde (figure below).
Download figure:
Standard image High-resolution imageThe analysis of the experimental data during the course of reaction showed that under the given conditions the catalytic activity did not change with time. Thus, all the experimental data can be used in kinetic calculations. Furthermore, the advantage of thin film catalyst is included in high efficiency and ease of recovery. The catalyst can be regenerated by treatment in air flow at  in 2 h.
in 2 h.
3.2. Reaction kinetics
According to the results obtained above, the thin films of P25-450-2 catalyst should be considered as typical catalyst for  xylene photooxidation in UV light. Therefore, the kinetics of reaction (6) was studied on P25-450-2 catalyst at conditions of ultraviolet irradiation.
xylene photooxidation in UV light. Therefore, the kinetics of reaction (6) was studied on P25-450-2 catalyst at conditions of ultraviolet irradiation.
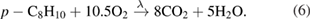
In the kinetic experiments the values of gas hourly space velocity on a volume basis (GHSV) varied from  to
to  and a catalyst film with the thickness of
and a catalyst film with the thickness of  was used. Under such conditions the influences of external as well as internal diffusions can be avoided. Indeed, in this range of flow rate the conversion extent decreased with increasing flow velocity, which indicates that there is no effect of external diffusion. The values of initial partial pressure of
was used. Under such conditions the influences of external as well as internal diffusions can be avoided. Indeed, in this range of flow rate the conversion extent decreased with increasing flow velocity, which indicates that there is no effect of external diffusion. The values of initial partial pressure of  xylene
xylene  , oxygen
, oxygen  and water vapor
and water vapor  varied in ranges: 2.1–11.3 hPa, 105–473 hPa, and 6–32 hPa, respectively. To study the effect of reaction products, carbon dioxide with the values of initial partial pressure
varied in ranges: 2.1–11.3 hPa, 105–473 hPa, and 6–32 hPa, respectively. To study the effect of reaction products, carbon dioxide with the values of initial partial pressure  from 0 to 24.89 hPa was added to the reaction mixture. The distance between the catalyst surface and the illuminating lamps is 7 cm. The intensity of UV light varied in range 650–1147 lux. In order to change the light intensity the nets of different mesh sizes are placed between the light source and the catalyst film. Under these conditions the conversion of
from 0 to 24.89 hPa was added to the reaction mixture. The distance between the catalyst surface and the illuminating lamps is 7 cm. The intensity of UV light varied in range 650–1147 lux. In order to change the light intensity the nets of different mesh sizes are placed between the light source and the catalyst film. Under these conditions the conversion of  xylene (X) varied from 0.10 to 0.98.
xylene (X) varied from 0.10 to 0.98.
The reaction rate on the studied catalysts was calculated by Temkin's formula [29]
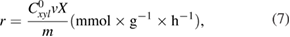
or
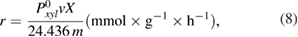
where  and
and  are the initial concentration
are the initial concentration  and initial partial pressure (hPa) of
and initial partial pressure (hPa) of  xylene in the gas mixture, respectively,
xylene in the gas mixture, respectively,  is the
is the  xylene conversion,
xylene conversion,  is the catalyst loading mass (g), and
is the catalyst loading mass (g), and  is the total flow velocity of reaction gas mixture
is the total flow velocity of reaction gas mixture  .
.
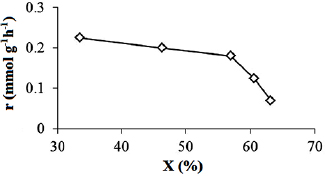
The convex shape of the curves reaction rate  versus conversion extent
versus conversion extent  on figure 13 shows that the reaction was inhibited by the substances participating in the reaction.
on figure 13 shows that the reaction was inhibited by the substances participating in the reaction.
Figure 13. Variation of reaction rate (r) with conversion extent (X) of  xylene at 40 °C on P25-450-2 thin film. Reaction conditions:
xylene at 40 °C on P25-450-2 thin film. Reaction conditions:  ;
;  ;
;  , and
, and  .
.
Download figure:
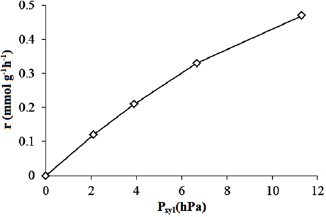
Standard image High-resolution imageThe dependence of the reaction rate on the partial pressures of substances involved in reactions is shown on figures 14 and 15. It can be seen from figures 14 and 15 that the graphs describing the dependence reaction rate  partial pressure of
partial pressure of  xylene
xylene  and oxygen
and oxygen  are gently arched in first case and almost linear in second case, that means probably the terms
are gently arched in first case and almost linear in second case, that means probably the terms  and
and  are present in both the numerator and the denominator of the equation, but the quantity of
are present in both the numerator and the denominator of the equation, but the quantity of  and
and  in denominator is relatively small or can be present only in the numerator of the equation with the reaction order of 1.
in denominator is relatively small or can be present only in the numerator of the equation with the reaction order of 1.
Figure 14. Variation of reaction rate  with partial pressure of p-xylene
with partial pressure of p-xylene  at
at  on P25-450-2 thin film. Reaction conditions:
on P25-450-2 thin film. Reaction conditions:  ;
;  ,
,  , and X = 0.4.
, and X = 0.4.
Download figure:
Standard image High-resolution imageFigure 15. Variation of reaction rate  with partial pressure of oxygen
with partial pressure of oxygen  on P25-450-2 thin film. Reaction conditions: T = 40 °C;
on P25-450-2 thin film. Reaction conditions: T = 40 °C;  ;
;  ;
;  , and X = 0.4.
, and X = 0.4.
Download figure:
Standard image High-resolution imageThe curves expressing the dependence of the reaction rate on concentration of water vapor (figure 16) have extreme shapes. At low concentrations of water vapor the reaction rate increases with the concentration of the given reactant, but reaches maximum values in the range of  , after that gradually decreases when the concentration of the considered reactant continues to grow.
, after that gradually decreases when the concentration of the considered reactant continues to grow.
Figure 16. Variation of reaction rate  with partial pressure of water vapor
with partial pressure of water vapor  on P25-450-2 thin film. Reaction conditions: T = 40 °C;
on P25-450-2 thin film. Reaction conditions: T = 40 °C;  ;
;  ;
;  , and X = 0.4.
, and X = 0.4.
Download figure:
Standard image High-resolution imageThis allows us to drive a common rule for the reaction that the concentration/pressure of water vapor is involved in both numerator and denominator of the kinetic equation, but the value of its exponent in the denominator must be greater than that in the numerator.
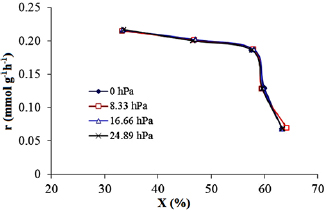
The coincidence of the curves showing the change of reaction rate with the degree of conversion (figure 17) at different  initial concentrations confirms that
initial concentrations confirms that  does not affect the reaction rate. The dependence of the reaction rate on the light intensity is shown in figure 18.
does not affect the reaction rate. The dependence of the reaction rate on the light intensity is shown in figure 18.
Figure 17. Variation of reaction rate  with conversion degree
with conversion degree  at different values of carbon dioxide initial partial pressure
at different values of carbon dioxide initial partial pressure  on P25-450-2 thin film. Reaction conditions: T = 40 °C;
on P25-450-2 thin film. Reaction conditions: T = 40 °C;  ;
;  ; and
; and  .
.
Download figure:
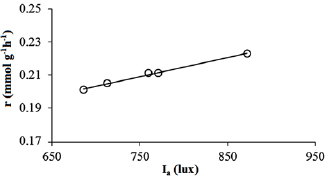
Standard image High-resolution imageFigure 18. Variation of reaction rate  with intensity of UV light irradiation
with intensity of UV light irradiation  on P25-450-2 thin film. Reaction conditions: T = 40 °C;
on P25-450-2 thin film. Reaction conditions: T = 40 °C;  ;
;  ;
;  ; and
; and  .
.
Download figure:
Standard image High-resolution imageFrom the presented above kinetic results the following conclusions should be drawn:
- The reaction is inhibited by the substances of reaction (
 xylene/oxygen/water) and the kinetic equation is not exponential;
xylene/oxygen/water) and the kinetic equation is not exponential; - The quantities
 and
and  can be present in both the numerator and the denominator of the equation, but quantity of
can be present in both the numerator and the denominator of the equation, but quantity of  and
and  in denominator is relatively small or present only in the numerator with exponent 1;
in denominator is relatively small or present only in the numerator with exponent 1; - Quantity
 is present both in the numerator and the dominator of the kinetic equation with its exponent in the numerator smaller than in the denominator;
is present both in the numerator and the dominator of the kinetic equation with its exponent in the numerator smaller than in the denominator; - Quantity
 does not participate in the kinetic equation.
does not participate in the kinetic equation. - Reaction rate is proportional to light intensity.
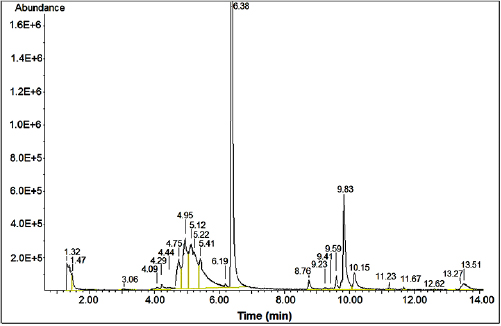
By-products formed during photocatalytic oxidation of  xylene were analyzed directly from the reaction gas mixture (figure 19) and showed that the by-product is 4-metylbenzadehyde presenting as traces. Thus, when considering the reaction kinetics the by-products could be ignored. In addition, the material equilibrium data for C also shows that only the product is
xylene were analyzed directly from the reaction gas mixture (figure 19) and showed that the by-product is 4-metylbenzadehyde presenting as traces. Thus, when considering the reaction kinetics the by-products could be ignored. In addition, the material equilibrium data for C also shows that only the product is  .
.
Figure 19. GC/MS Spectrum of intermediate products of reaction gas mixture.
Download figure:
Standard image High-resolution imageFor the rates of photo-oxidation of chloroform in aqueous suspension of  the following kinetic equation has been proposed by Kormann et al [30]:
the following kinetic equation has been proposed by Kormann et al [30]:
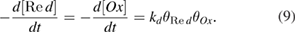
In this equation quantity  and
and  respectively are the fraction of adsorption of the electron-donating reductant and electron-accepting oxidant to the catalyst surface, and
respectively are the fraction of adsorption of the electron-donating reductant and electron-accepting oxidant to the catalyst surface, and  is reaction rate constant.
is reaction rate constant.
Because  of P25-450-2 catalyst is 3.155 eV, under the irradiation of UV light, its electron was excited from valence band to the conduction band
of P25-450-2 catalyst is 3.155 eV, under the irradiation of UV light, its electron was excited from valence band to the conduction band  generating a positive hole in the valence band
generating a positive hole in the valence band 
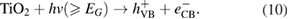
Next, hydroxyl radicals  , a strong oxidizing agent, are formed by oxidation of water molecules adsorbed on the surface of
, a strong oxidizing agent, are formed by oxidation of water molecules adsorbed on the surface of  and –OH group (as seen in IR spectra) by photo-generated holes [31]:
and –OH group (as seen in IR spectra) by photo-generated holes [31]:
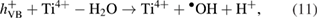
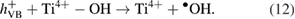
Electrons in the conduction band can be trapped by molecular oxygen adsorbed on the surface of  P25 to form superoxide radical anion
P25 to form superoxide radical anion  [31]:
[31]:
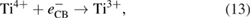
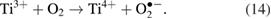
Further, these powerful oxidants (hydroxyl radicals and reactive oxygen species) participate in the reaction with organic compounds to form  and
and  [32]:
[32]:
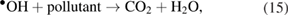
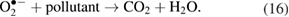
So, oxidant in the case of photo-oxidation in gas phase is both  forming from dissociative adsorbed water and
forming from dissociative adsorbed water and  generating from adsorbed molecular oxygen. Then,
generating from adsorbed molecular oxygen. Then,  in this case equal
in this case equal

When considering the near-linear dependence of the reaction rate on the partial pressure of  xylene, the question is whether the reaction proceeds according to the Langmuir-Hinshelwood or Eley-Rideal mechanisms? Turchi and Ollis [31] studied two reaction schemes in which the authors considered either the attack of an adsorbed hydroxyl radical on an adsorbed organic substrate or the attack of given radical on a free organic substrate as the rate-determining steps. In our case, with a small amount of catalyst (15 mg) and a low concentration of
xylene, the question is whether the reaction proceeds according to the Langmuir-Hinshelwood or Eley-Rideal mechanisms? Turchi and Ollis [31] studied two reaction schemes in which the authors considered either the attack of an adsorbed hydroxyl radical on an adsorbed organic substrate or the attack of given radical on a free organic substrate as the rate-determining steps. In our case, with a small amount of catalyst (15 mg) and a low concentration of  xylene
xylene  , but a high concentration of oxygen
, but a high concentration of oxygen  in the reaction stream, the catalyst surface should be completely covered with adsorbed oxygen and only a limited number of
in the reaction stream, the catalyst surface should be completely covered with adsorbed oxygen and only a limited number of  xylene molecules which scarcely access to the surface, can participate in the reaction. Therefore, the reaction rate should be proportional to the surface concentration of
xylene molecules which scarcely access to the surface, can participate in the reaction. Therefore, the reaction rate should be proportional to the surface concentration of  xylene. In principle, the participation of
xylene. In principle, the participation of  xylene in the reaction from the gas phase cannot be ruled out; in this case, the order of
xylene in the reaction from the gas phase cannot be ruled out; in this case, the order of  xylene concentration in the kinetic equation is equal 1. But there is a relationship between the quantity of adsorbed
xylene concentration in the kinetic equation is equal 1. But there is a relationship between the quantity of adsorbed  xylene and its conversion extent, so the first hypothesis should be more appropriate. On P25-450-4 sample the quantity of adsorbed
xylene and its conversion extent, so the first hypothesis should be more appropriate. On P25-450-4 sample the quantity of adsorbed  xylene and its conversion extent were reached
xylene and its conversion extent were reached  and 99% respectively, while on the sample, calcined at 550 °C in 2 h (P25-550-2) these values were
and 99% respectively, while on the sample, calcined at 550 °C in 2 h (P25-550-2) these values were  and 87%, respectively. Since the reaction follows the Langmuir-Hinshelwood model, in which the attack of an adsorbed hydroxyl radical on an adsorbed organic substrate [31] and the reactions (15) and (16) are the rate-determining steps, the rate of reaction (6) could be written in a following form
and 87%, respectively. Since the reaction follows the Langmuir-Hinshelwood model, in which the attack of an adsorbed hydroxyl radical on an adsorbed organic substrate [31] and the reactions (15) and (16) are the rate-determining steps, the rate of reaction (6) could be written in a following form
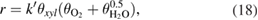
where  is the rate constant,
is the rate constant,  and
and  are the fraction of the
are the fraction of the  xylene and oxygen molecules adsorbed to the catalyst surface, respectively,
xylene and oxygen molecules adsorbed to the catalyst surface, respectively,  is the fraction of the water dissociative adsorbed to the surface.
is the fraction of the water dissociative adsorbed to the surface.
Mills et al [33] indicated that the constant of proportionality  of the photo-mineralization of organic substrates
of the photo-mineralization of organic substrates  by oxygen on
by oxygen on  catalyst in following kinetic equation
catalyst in following kinetic equation
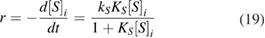
is proportional to the rate of light absorption  as well as the fraction of
as well as the fraction of  non-competitive adsorption at
non-competitive adsorption at  sites. In this equation
sites. In this equation  is the reaction rate,
is the reaction rate,  is the kinetic constant,
is the kinetic constant,  and
and ![${{[ S ] }_{i}}$](https://content.cld.iop.org/journals/2043-6262/9/4/045006/revision1/ansnaaed2eieqn218.gif) respectively are the adsorption equilibrium constant and concentration of substrate
respectively are the adsorption equilibrium constant and concentration of substrate  . The exponent
. The exponent  was 0.5 when the light intensity was high but equal to 1 at low light intensity values. Therefore, the kinetic equation (18) can be written as
was 0.5 when the light intensity was high but equal to 1 at low light intensity values. Therefore, the kinetic equation (18) can be written as
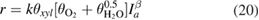
where  is the degree of coverage of the respective species and
is the degree of coverage of the respective species and  is total light intensity.
is total light intensity.
The fraction of adsorption of surfactants to the surface is determined by the following Langmuir formulae
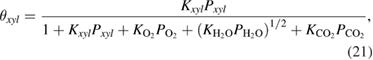
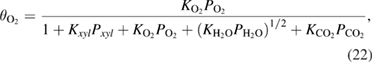
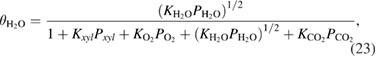
where  is the Langmuir adsorption constant of the
is the Langmuir adsorption constant of the  species on the surface of the catalyst and
species on the surface of the catalyst and  is their partial pressure. Then, the final expression of the kinetics for the whole process of
is their partial pressure. Then, the final expression of the kinetics for the whole process of  xylene photooxidation could be described as follows
xylene photooxidation could be described as follows
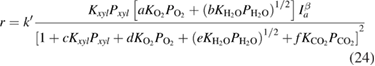
where  are the constants.
are the constants.
Defining  ,
,  ,
,  ,
,  ,
,  ;
;  ,
,  , and equation (24) can be written by follow common equation, in accordance with the above conclusions of the reaction kinetics:
, and equation (24) can be written by follow common equation, in accordance with the above conclusions of the reaction kinetics:
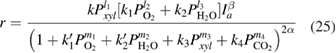
where  is reaction rate of
is reaction rate of  xylene deep photooxidation;
xylene deep photooxidation;  are constants of the kinetic equation;
are constants of the kinetic equation;  are partial pressure of the corresponding components;
are partial pressure of the corresponding components;  is total light intensity;
is total light intensity;  and
and  are reaction orders of the corresponding reactants and light intensity; and
are reaction orders of the corresponding reactants and light intensity; and  is surface coverage.
is surface coverage.
To determine the reaction order and the values of kinetic coefficients, the solver tool in MS excel was used to calculate the experimental data in combination with the following conditions:  (step 0.25),
(step 0.25),  (step 0.25),
(step 0.25),  (step 0.25),
(step 0.25),  .
.
The calculated results gave the best coincidence with the experimental data when:  ,
,  ;
;  ;
;  ;
;  and
and  . The calculation also showed that
. The calculation also showed that  . Therefore, the kinetic equation for the reaction can be written as follows
. Therefore, the kinetic equation for the reaction can be written as follows
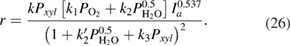
The values of the kinetic constants in equation (26) are presented in table 2.
Table 2. Values of the kinetic constants in equation (26).
| Kinetic constants | Unit | Value |
|---|---|---|
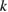 |
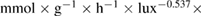 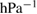 |
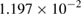 |
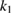 |
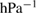 |
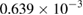 |
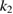 |
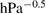 |
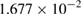 |
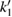 |
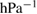 |
0 |
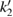 |
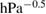 |
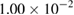 |
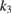 |
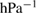 |
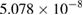 |
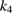 |
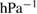 |
0 |
| Variance | % |  |
As it follows from table 2, the value of  is very small in comparison with
is very small in comparison with  and equation (26) becomes
and equation (26) becomes
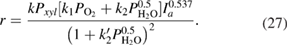
The form of equation (26) showed that, the reaction takes place in the areas of high coverages. The exponent value  of light intensity in kinetic equation (26) is found to be 0.537. According to the authors [17–19], the order of photon flux
of light intensity in kinetic equation (26) is found to be 0.537. According to the authors [17–19], the order of photon flux  for photocatalytic degradation of trichloroethylene in gas-phase over
for photocatalytic degradation of trichloroethylene in gas-phase over  supported on glass bead depends on the intensity and wavelength of light, varying from 0.42 to 1.0. The square-root dependence of reaction rate of chloroform photodegradation in the presence of
supported on glass bead depends on the intensity and wavelength of light, varying from 0.42 to 1.0. The square-root dependence of reaction rate of chloroform photodegradation in the presence of  on light intensity was reported by Kormann et al [30]. This relationship was explained by the role of hydroxyl radical in the capture of holes and the initiator of oxidation of electron-donating substrates. In our investigation, as it follows from table 2, the value of coefficient
on light intensity was reported by Kormann et al [30]. This relationship was explained by the role of hydroxyl radical in the capture of holes and the initiator of oxidation of electron-donating substrates. In our investigation, as it follows from table 2, the value of coefficient  of equation (26) is higher than
of equation (26) is higher than  about 28 times.
about 28 times.
The extreme form of the dependence of the reaction rate on the partial pressure of water vapor, as shown in figure 16, is the result of the squared of the denominator of equation (26). When the concentration of water vapor  is low, i.e.
is low, i.e.  in the denominator of equation (26) is negligible compared to 1 and it follows that r is proportional to
in the denominator of equation (26) is negligible compared to 1 and it follows that r is proportional to  , that means the reaction order of water vapor is +0.5. At high partial pressures of water vapor, the degree of surface coverage
, that means the reaction order of water vapor is +0.5. At high partial pressures of water vapor, the degree of surface coverage  is high, i.e.
is high, i.e.  in the denominator of equation (26) is much higher than 1 and the rate equation is proportional to
in the denominator of equation (26) is much higher than 1 and the rate equation is proportional to  , that means the reaction order of water vapor is −0.5. It was reported [13] that the concentration of water vapor in the gas mixture initially favored the photocatalytic process, but an increase in the water content reduced the rate of removal of acetone for a longer reaction time.
, that means the reaction order of water vapor is −0.5. It was reported [13] that the concentration of water vapor in the gas mixture initially favored the photocatalytic process, but an increase in the water content reduced the rate of removal of acetone for a longer reaction time.
Therefore, the chosen kinetic model should be based on the Langmuir-Hinshelwood mechanism, in which the interaction between adsorbed  xylene with molecular chemisorbed oxygen
xylene with molecular chemisorbed oxygen  and radical
and radical  is a rate-determining step.
is a rate-determining step.
All the points of view discussed above also lead to the conclusion that the nature of the catalytic action for photooxidation of  xylene on
xylene on  should be considered as a heterogeneous catalytic reaction involving
should be considered as a heterogeneous catalytic reaction involving  and free radicals
and free radicals  generated under the light irradiation.
generated under the light irradiation.
4. Conclusion
It can be concluded that thermal treatment has a meaningful effect on  degussa P25 properties and performance. Calcination at
degussa P25 properties and performance. Calcination at  for 2 h is able to create
for 2 h is able to create  of small nanoparticle size and low band gap energy, causing it to be a highly active and stable bare
of small nanoparticle size and low band gap energy, causing it to be a highly active and stable bare  catalyst for gas-phase photooxidation of
catalyst for gas-phase photooxidation of  xylene under ultraviolet light. The kinetic equation (26) based on Langmuir-Hinshelwood mechanism is the kinetic equation for gas-phase photooxidation reaction of
xylene under ultraviolet light. The kinetic equation (26) based on Langmuir-Hinshelwood mechanism is the kinetic equation for gas-phase photooxidation reaction of  xylene on
xylene on  catalysts under UV light radiation. On
catalysts under UV light radiation. On  , the photoreaction proceeds in the regions of high coverages;
, the photoreaction proceeds in the regions of high coverages;  xylene and oxygen participates in the reaction in molecular adsorbed phase; water vapor is involved in the reaction in the forms of
xylene and oxygen participates in the reaction in molecular adsorbed phase; water vapor is involved in the reaction in the forms of  and
and  . The reaction rate is proportional to concentration of
. The reaction rate is proportional to concentration of  xylene and oxygen. The effect of water vapor on the reaction rate is positive in the region of low concentrations and is negative in the high-concentrations region.
xylene and oxygen. The effect of water vapor on the reaction rate is positive in the region of low concentrations and is negative in the high-concentrations region.
Acknowledgment
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2016.01.