Abstract
This paper utilized urea functionalized multiwalled carbon nanotubes fertilizer as plant nutrition for rice to understand fully their mechanism of interaction. Surface modification of multiwalled carbon nanotubes was treated by nitric acid at different reflux times. The individual and interaction effects between the design factors of functionalized multiwalled carbon nanotube amount and functionalization reflux time with the corresponding responses of nitrogen uptake and nitrogen use efficiency were structured via the Response Surface Methodology based on five-level central composite design. The urea functionalized multiwalled carbon nanotubes fertilizer with optimized 0.5 weight% functionalized multiwalled carbon nanotubes treated at 21 h of reflux time achieve tremendous nitrogen uptake at 1180 mg/pot and NUE up to 96%. The FT-IR results confirm the formation of acidic functional groups of functionalized MWCNTs and UF-MWCNTs. The morphological observation of transmission electron microscopy shows extracellular regions to be the preferred localization of functionalized multiwalled carbon nanotubes in fresh plant root cells independent of their size and geometry. Penetration into the plant cell results in breaching of graphitic tubular structure of functionalized multiwalled carbon nanotubes with their length being shortened until ∼50 nm and diameters becoming thinner until less than 10 nm. The capability to agglomerate after translocation into the plant cells alarms potential cytotoxicity effect of functionalized multiwalled carbon nanotubes in agriculture. These work findings have suggested using urea functionalized multiwalled carbon nanotubes for effective nutrient delivery systems in rice plant.
Export citation and abstract BibTeX RIS
1. Introduction
The elementary composition of nanomaterials used with fertilizers in nanoagriculture can be categorized into metals or metal oxides (silver [1], nano titanium oxide [2, 3]), metalloids (silica [4]), non-metals (sulphur [5, 6] and carbon (MWCNTs [7, 8]). MWCNTs as efficient molecular transporter for plant cell walls [9] act as nutrient delivery system that improves the soil environment and promotes crop growth metabolism [7, 10–12]. Thus MWCNTs in pristine and functionalized forms have been widely studied in the past decades, and received growing attention recently because of the rising needs for efficient nutrient utilizing from fertilizer application as plant nutrition for crops [13, 14].
Inefficient plant nutrition was reported for nitrogenous fertilizer because 50 to 70% is lost through leaching and releasing to the atmosphere [15] due to unstable nitrogen in the form of nitrate or ammonium ions that leads to low NU, low NUE [16, 17], unfavourable environmental impacts cause eutrophication phenomena and higher operational cost to farmers. MWCNTs are combined with nitrogenous fertilizer to increase their efficiency as plant nutrition. However, pristine MWCNTs surfaces are chemically inactive to capably combine with fertilizer. MWCNTs tend to be an agglomerated material that will bundle together, causing many site defects [18] and are cytotoxic to the living cells in the environment [19]. Functionalization of the MWCNTs is an important method utilized for introduced active functional groups, which represent useful sites, aiming to separate them into individual nanotubes, to improve the solubility and compatibility, thus activating their surfaces [20].
The ability of MWCNTs based nutrient delivery system for plant tissue penetration to encourage NU and NUE depends strongly on the physical and chemical properties of functionalized-MWCNTs individually [21, 22]. Although the transportation in the plant cell is complicated due to the extra barrier in the structure in conjunction with the cell membrane existence, the functionalized-MWCNTs are reported to be able to penetrate into the plant cell wall of paddy [23], tomato [24], mustard seeds [25] and gram plants [26] to perform as efficient nutrient delivery system. Improvement of water uptake into the plant cells has been implied as the growth stimulator. Until now, the entry and localization mechanism of the functionalized-MWCNTs in the plant cells are still vague and raise contradict explanation among researchers [27, 28]. Therefore, further examination on mechanism of interaction at the cellular level is critically important for enabling successful applications of functionalized-MWCNTs based nutrient delivery system in fertilizer application.
In this work significantly improved NU and NUE of urea fertilizer can be achieved via chemically nitrogen functionalized-MWCNTs application to generate efficient nitrogen delivery system for paddy. An optimal nitric acid treatment process is able to chemically activate the pristine MWCNTs surfaces with various carboxyl functional groups and compatible to attach with nitrogen from urea fertilizer to become urea functionalized-MWCNTs (UF-MWCNTs). The UF-MWCNTs fertilizer applied as 15N isotope to capable amount of nitrogen usage by paddy derived from the fertilizer (NDFF), which is traceable and measured, provides valuable information on their advantages in significantly improving the nitrogenous fertilizer performance more than 90%. This research work mainly highlighted the mechanism of interaction at the cellular level which was revealed using electron microscopy morphological analysis approach.
2. Materials and methods
2.1. Synthesis of UF-MWCNTs fertilizer
Chemical vapor deposition growing MWCNTs (purity of 95%, outside diameter of 10–20 nm, inside diameter of 5–10 nm, and length ranging from 0.5 μm to 1.0 μm) was purchased from Cahaya Tech (M) Private Ltd Analytical chemical grade (Merck) of nitric acid (HNO3) (69%) was used as received. MWCNTs was refluxed with nitric acid at 12, 15, 18, 21, and 24 h, and the suspension was cooled down to room temperature. The solution of functionalized-MWCNTs was vacuum-filtered, washed to remove excess HNO3 and further dried in a vacuum oven for 2 h. Various amounts (0.1–0.6 wt%) of functionalized-MWCNTs were stirred together with urea for 6 h at 150 rpm to produce UF-MWCNTs and dried at 70 °C for 5 h.
2.2. Fertilizer application and agronomic practice
A pot experiment was conducted in a glasshouse. During the early rice growing period, water level was kept at 1–2 cm above the soil surface and 10 cm at the later growth period. Rice seeds were pre-germinated before transferred into the pots with flooded soil taken from the rice field. Recommended rates of 120 kg hm−2 N−1 (Urea fertilizer), 50 kg hm−2 P2O5, and 50 kg hm−2 K2O were used. UF-MWCNTs were applied for the N source in three separation times (15, 20 and 35 days after transplanting). Triple superphosphate (Phosphorus (P) source) and muriate of potash (potassium (K) source) were applied as basal fertilizer. Insect, pest, disease and weed control were done according to standard agronomic practices.
2.3. Optimization by Response Surface Methodology (RSM)
CCD of RSM is used to define the optimum level of design parameters for UF-MWCNTs fertilizer production. The different weight% (wt%) of functionalized-MWCNTs and functionalization reflux time were assessed at five levels: −1.682, −1, 0, +1, and +1.682 as presented in table 1. A total of 13 experiments (table 2) were conducted with corresponding maximum responses NUE and NU with three replications for every set of trials. The Design Expert 9 (Stat Ease, Inc., Minneapolis, MN, USA), was used for the regression analysis of the experimental data and to plot the three-dimensional (3D) response surface graphs to illustrate the relationship between the responses and the experimental levels of each design factor and the optimum levels of the selected variables.
Table 1. Experimental codes and levels of independent variables for RSM optimization.
| Levels | ||||||
|---|---|---|---|---|---|---|
| Variables | Units | −1.682 | −1 | 0 | +1 | +1.682 |
| MWCNTs, X1 | wt% | 0.1 | 0.2 | 0.3 | 0.5 | 0.6 |
| Reflux time, X2 | Hour | 12 | 15 | 18 | 21 | 24 |
Table 2. Experimental design matrix.
| Run | X1 | X2 |
|---|---|---|
| 1 | 0.6 | 18 |
| 2 | 0.3 | 18 |
| 3 | 0.3 | 24 |
| 4 | 0.3 | 18 |
| 5 | 0.3 | 18 |
| 6 | 0.3 | 12 |
| 7 | 0.5 | 21 |
| 8 | 0.1 | 15 |
| 9 | 0.2 | 18 |
| 10 | 0.1 | 21 |
| 11 | 0.3 | 18 |
| 12 | 0.5 | 15 |
| 13 | 0.3 | 18 |
2.4. Nitrogen uptake and use efficiency analysis of UF-MWCNTs
UF-MWCNTs fertilizer labelled with 15N used was ISOTEC™ Urea  5% atom excess (a.e.) is used and the amount of N fertilizer that a plant has taken up is determined. The plant samples for 15N were analyzed by using emission spectrometer detector (NO17), FAN (Fischer ANalysen Instrumente), Germany. Nitrogen fertilizer utilization by crops and retention in the soil were calculated using 15N direct technique following equations (1)–(4) [29].
5% atom excess (a.e.) is used and the amount of N fertilizer that a plant has taken up is determined. The plant samples for 15N were analyzed by using emission spectrometer detector (NO17), FAN (Fischer ANalysen Instrumente), Germany. Nitrogen fertilizer utilization by crops and retention in the soil were calculated using 15N direct technique following equations (1)–(4) [29].
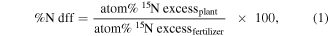



where N dff is fraction of N in the plant derived from the 15N labelled fertilizer.
2.5. Morphological analyses of functionalized-MWCNTs
The separated fresh paddy's root tissue (<1 cm3) structure was preserved by being coated with a fixing solution of 5% glutaraldehyde and 4% paraformaldehyde, before evaporated thoroughly using a vacuum pump. After 2 days, the glutaraldehyde was removed, and replaced with 2% osmium tetroxide followed by rinsing with 0.1 M PBS buffer (pH = 7.2). After rinsing with gradient ethanol five times every 20 min for dehydration, 0.25 ml of epoxy was added and refreshed three times every 2 h. The rice plant tissue then stood for 12 h before embedding into Spurr's resin. Finally, Leica ultra-cut was used to cut thin pieces of rice plant tissue, before cooled to −100 °C using liquid nitrogen. Sections were mounted on copper grids, and later the samples were examined via HT-7700 TEM using a voltage of 200 Kv, till 500 00x magnification.
3. Results and Discussion
3.1. Treatment efficiency evaluation
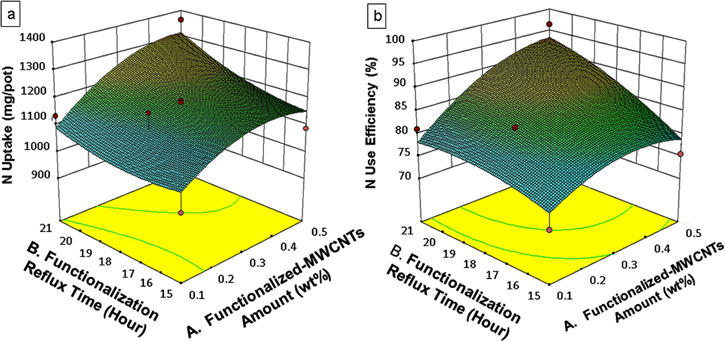
The optimum conditions for UF-MWCNTs performances manipulating NU and NUE of paddy were determined by means of the Central Composite Design (CCD) of RSM. The optimization process desired goals for each response (NU and NUE) were chosen to be maximized to achieve the highest performance of UF-MWCNTs fertilizer. The obtained responses revealed in table 3 were varied between 978 to 1363 mg/pot (NU) and 71 to 96% (NUE). The 3D response surface plots of RSM depicted in figure 1 show that the maximum NU (1363.55 mg/pot) and NUE (96.35%) by paddy for UF-MWCNTs occurred at condition of 0.50 wt% of functionalized-MWCNTs amount used treated at 21 h. of functionalization reflux time. The RSM further confirmation report (table 4) based on the 95% confidence level presents the optimum design factors (0.5 wt% functionalized-MWCNTs and 21 h. functionalization reflux time) for the development of UF-MWCNTs fertilizer.
Table 3. Results of CCD.
| Run | Response 1 NU | Response 2 NUE |
|---|---|---|
| 1 | 1182.12 | 86.29 |
| 2 | 1100.00 | 86.16 |
| 3 | 1344.75 | 85.20 |
| 4 | 1100.00 | 85.00 |
| 5 | 1181.60 | 86.25 |
| 6 | 1256.30 | 83.85 |
| 7 | 1363.55 | 96.35 |
| 8 | 977.84 | 71.38 |
| 9 | 1185.02 | 86.50 |
| 10 | 1134.47 | 81.09 |
| 11 | 1187.41 | 86.67 |
| 12 | 1087.31 | 75.46 |
| 13 | 1189.00 | 90.00 |
Figure 1. 3D response surface plots of NU (a) and NUE (b) of paddy as a function of functionalized-MWCNTs (wt%) and functionalization reflux time (hour).
Download figure:
Standard image High-resolution imageTable 4. Optimization results for design factors of UF-MWCNTs fertilizer.
| Factors | Name | Level | Low level | High level |
|---|---|---|---|---|
| X1 | Functionalized-MWCNTs | 0.50 | 0.10 | 0.50 |
| X2 | Functionalization reflux time | 21.00 | 15.00 | 21.00 |
Confidence level = 95%.
Application of UF-MWCNTs fertilizer enhances the NU and NUE responses significantly. The NU and NUE achieved by the paddy increased with functionalized-MWCNTs treatment time up to 21 h. While further increment results in NU and NUE decrement. The competency of NU and NUE from UF-MWCNTs fertilizer by paddy also increased with amount of functionalized-MWCNTs and dropped after 0.6 wt% functionalized-MWCNTs was added. This shows that the performance of UF-MWCNTs fertilizer is proportional to the amount of functionalized-MWCNTs usage and functionalization time until the optimal value of 0.5 wt% and 21 h of functionalization time.
This result can be explained that higher amount of functionalized-MWCNTs grafted with urea fertilizer indicates more available nitrogen in the soil to be absorbed by paddy and 21 h functionalization reflux time appears to be the optimum treatment condition that sustains the molecular transport capability of functionalized-MWCNTs. Hashim et al [16] reported that the NUE of Malaysian local rice variety, under standard UF treatment reached up to 50% only. The remaining 50% of N from the applied fertilizer either remained in the soil or was lost to the environment [30]. Noticeable improvement on NUE using UF-MWCNTs fertilizer is evident on the efficient nitrogen delivery system to paddy, leads to substantial solution for inefficient plant nutrition reported for nitrogenous fertilizer. It is conclusive at this point that the developed UF-MWCNTs fertilizer using the optimum values of designed parameters dictates the performance of UF-MWCNTs fertilizer. Further analysis for morphological studies was performed to understand its mechanism of interaction.
3.2. Confirmation of functionalized-MWCNTs uptake into plant roots
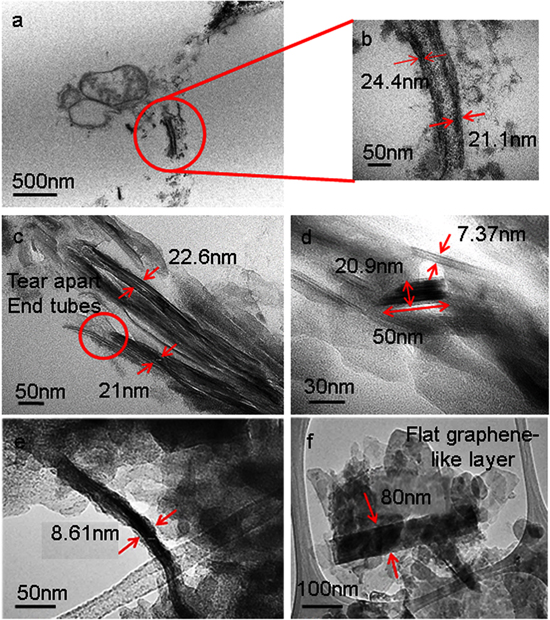
The uptake of functionalized-MWCNTs by plant root cells of paddies from UF-MWCNTs fertilizer was confirmed by the TEM micrographs captured during early (5 weeks) and end growth (13 weeks) stages (figure 2). These direct evidences proved the uptake of functionalized-MWCNTs by paddy roots occurred at soil-plant interactions throughout the paddy life continuously. However, the structure of the uptake functionalized-MWCNTs seemed to change drastically compared to aligned pristine MWNTs. Even the tubular structure of functionalized-MWCNTs can still be clearly seen with an outside diameter of ∼20 nm in range (figure 2(a)), but with higher magnification of TEM, figure 2(b) depicts most of the aligned multiwall structures being ruptured. Most of the functionalized-MWCNTs were found to be separated into individual nanotubes with their end tear apart leaving them with irregular edges (figure 2(c)). The long functionalized-MWCNTs length ranging from 0.5 μm to 1.0 μm were shortened until ∼50 nm in length (figure 2(d)) and became thinner to a point that their diameters were less than 10 nm (figure 2(e)). Significant finding concerning geometry change of functionalized-MWCNTs was revealed in figure 2(f) when a flat graphene-like layer with a width of ∼80 nm was found in the cell of paddy root samples. This might be correlated with the open tubular functionalized-MWCNTs structure into a flat graphene layer with uniform ended edges.
Figure 2. TEM micrographs showing the uptake of functionalized-MWCNTs into paddy root, resulting in most of the aligned multiwall structures being ruptured (a-b), separated into individual nanotubes with irregular edges (c), cut into short (d), thinner (e) and flat graphene-like layer (f) MWCNTs.
Download figure:
Standard image High-resolution imageThese observations confirmed the uptake of functionalized-MWCNTs into the plant cells, and the process was found to have destroyed most of the graphitic structure of the functionalized-MWCNTs. This is significant as nanoagriculture aims to avoid stable graphitic structure and agglomeration properties of MWCNTs that are often related to their toxic properties to plant cells. The direct evidence of the physical uptake of functionalized-MWCNTs through phospholipid bilayers into plant roots in control environment has been widely studied [31–34], but is still lacking involving direct soil-plant interactions. Similar observations were made by Serag et al [35] on the size-dependent cellular uptake and distribution of CNTs in plant cells. Short MWCNTs (30–100 nm) was suggested to have a stronger ability to penetrate protoplast plasma membranes, since the uptake of MWCNTs into plant cells was reported with <100 nm in length. The helical geometry of MWCNTs would allow a smooth transition through the bilayers like screw-driving. However, surface patterning was said to have a bigger effect on the translocation than the geometry of uptake functionalized-MWCNTs [36]. MWCNTs surface patterning may occur naturally through the spontaneous self-assembly of biomolecules onto their surface facilitating their translocation through cell membranes and resulting in the opening of the helical and tubular structures of the functionalized-MWCNTs into a flat graphene layer [37]. Thus, it indicates that functionalized-MWCNTs in UF-MWCNTs fertilizer were taken up by the plant roots and localized in the plant root cells with destroyed graphitic multiwall structure.
3.3. Localization of functionalized-MWCNTs in the plant root cells
The localization of functionalized-MWCNTs in paddy root cells was further investigated (figure 3). Functionalized-MWCNTs are preferentially localized in the extracellular regions, along the cell walls (figures 3(a), (b)). Short functionalized-MWCNTs were found to be localized deeper inside the subcellular region of plant cells (figure 3(c)). While longer functionalized-MWCNTs were found to be able to penetrate the plasma membrane and localized near the cell wall (figure 3(d)). The similar features of MWCNTs uptake by the plant cells were reported elsewhere [35, 38].
Figure 3. Preferential localization of functionalized-MWNTs along extracellular regions of plant cell (a), (b) with short functionalized-MWNTs deeper inside the subcellular region (c) and long functionalized-MWNTs near undamaged cell wall of plant cells (d).
Download figure:
Standard image High-resolution imageLocalization of functionalized-MWCNTs in the extracellular regions and along the extracellular regions of cell walls is largely attributable to the similar way of nutrient entry, through mass flow route along the plant cell wall. In contrast, Yaron et al [39] reported the preferential localizations for pristine MWCNTs are within the subcellular region of plant cells only through the endocytosis method instead of the extracellular regions with direct penetration. Localization of functionalized-MWCNTs near the undamaged plant cell wall structures might be correlated with translocation of these functionalized-MWCNTs from other entries of plant cell walls instead of penetration through the near cell wall. Serag et al [35] reported that short MWCNTs were only observed inside vacuoles, nucleus and plastids. They suggested that the size-dependent cellular uptake of MWCNTs into cellular structures was significant and revealed a great potential for MWCNTs to be used effectively for molecular cargo delivery into specific targets of compartments.
However, this study proved that stronger effect for penetration and localization of functionalized-MWCNTs in the subcellular region of plant cells is shown by MWCNTs surface patterning through functionalization regardless of their size. To gain insight into the size factors governing the functionalized-MWCNTs localization in plant cells, observation was made with reference to functionalized-MWCNTs shape and geometry in plant cells.
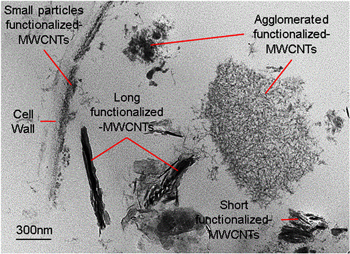
Localization of functionalized-MWCNTs in the plant cells was observed to be independent of their shape and geometry (figure 4). Functionalized-MWCNTs in irregular short multiwall, small particles and individual nanotube structures were observed to be localized inside the subcellular region of plant cell near to each other. Accumulation of functionalized-MWCNTs occurred outside the leaking plant organelles instead of penetrating and destroying the plant organelle features. Therefore, it can now be suggested that functionalized-MWCNTs that are localized in the plant root cells have the possibility to agglomerate with each other, alarming their usage in nanoagriculture.
Figure 4. Localization of functionalized-MWCNTs in the plant cells is independent of their size and geometry at the same location.
Download figure:
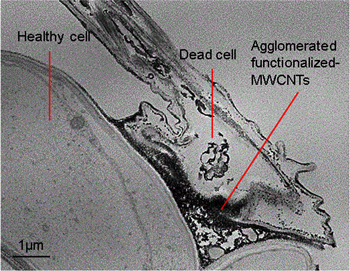
Standard image High-resolution imageFurther TEM investigation on the functionalized-MWCNTs localization in the extracellular region (figure 5) described the restriction of the uptake functionalized-MWCNTs into the subcellular region of plant cells. Most of the plant cells remain healthy with fine cell walls and plasma membrane structures. However, some of the plant cells are dead with functionalized-MWCNTs agglomerated inside. Similar observations were reported by Tan and Fugetsu [40] who had mentioned that the cell walls of the rice cell suspension restricted the entry of the MWCNTs into cellular cytoplasm, and demonstrated a self-defence response by sacrificing a small population of cells that aggregate with the nanomaterials to protect the remainder of the cells in the culture. Plant defence system aims to avoid translocation of foreign materials including functionalized-MWCNTs to the top part of paddy plant and produce safe grains for food.
Figure 5. TEM micrographs on plant cells reveal dead and healthy plant cells.
Download figure:
Standard image High-resolution imageThe cell death might be due to the penetration of functionalized-MWCNTs into the subcellular region as compared to healthy cells without any functionalized-MWCNT features. This strongly suggests that functionalized-MWCNTs localized within a few plant cells might resultg in the cell death but be prevented from penetrating many other cells to leave them safe and healthy. The nanometer size and the concentration of the MWCNTs were responsible for potential cytotoxicity such as cell death or necrosis identified by leakage of cytoplasmic content as a result of membrane disruption [19]. Thus, functionalization treatment and amount of functionalized-MWCNTs used are significant factors to avoid toxicity effects on plant cells.
3.4. Mechanism of functionalized-MWCNTs entry into plant roots
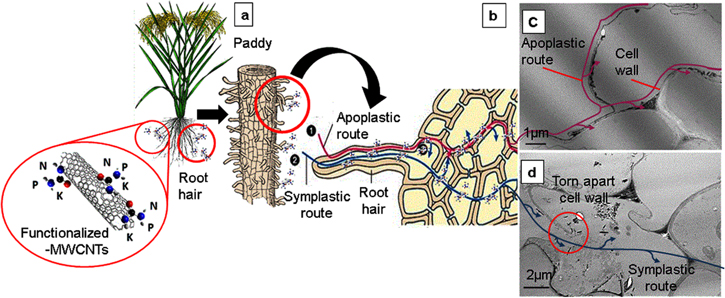
The functionalized-MWCNTs localization is significantly related to the same route of nutrient uptake by plant root namely mass flow type of mechanism as illustrated in figure 6. In soil, mass flow is the most prominent way for nutrient uptake and it involves the nutrients moving with the water flow into the plant roots (figure 6(a)). There are two routes of water flow; apoplastic and symplastic. The apoplastic route is the cell wall route until the xylem, while the symplastic route enters the root cells to the xylem (figure 6(b)). Localization of functionalized-MWCNTs along the plant cell wall is related to the apoplastic routes (figure 6(c)) of the mass flow mechanism and the penetration of some functionalized-MWCNTs through the plasma membrane into the plant cells is associated with symplastic routes (figure 6(d)) of the mass flow mechanism. Some parts of the cell wall were torn apart (in the red circle) as a result of direct penetration by larger functionalized-MWCNTs as compared to smaller functionalized-MWCNTs along the plant cell wall.
Figure 6. Mass flow mechanism of f-MWCNTs entry into the paddy plant root (a) [41] to the root hair cells (b) [42] through apoplastic route (c) and symplastic route (d).
Download figure:
Standard image High-resolution imageThis finding reinforced that smaller MWCNTs are subjected to easier penetration into plant roots [31], but surface patterning (functionalization) has a bigger effect on the translocation through the plant cell wall [37]. The functionalized-MWCNTs with the nutrients grafted on their surfaces might be absorbed together during the process of nutrient uptake by plants by sheer vacuum.
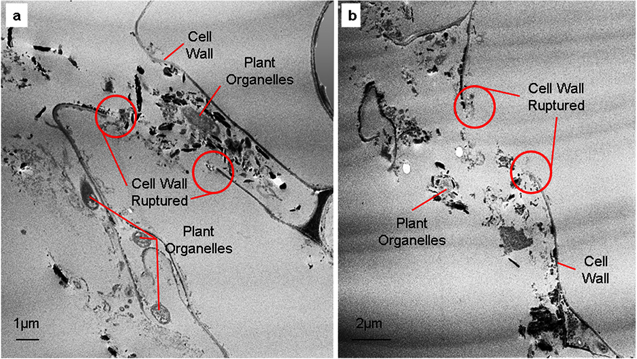
The relationship of the MWCNTs delivery system with water flow was described when the plant germination rate was reported to be improved through efficient intake of water, oxygen and nutrients [43, 44]. Hence, leaching and losses of nutrients to unintended targets like soil are reduced. Recently, there are two theories regarding the mechanism of MWCNTs entry into plants; (1) direct penetration by creating new pores and (2) endocytosis. Direct penetration is the invasion of the MWCNTs into epidermal cells (figure 7) as described by Melotto et al [27] in their review regarding direct penetration by pathogen into plant cells. Direct penetration has been described widely concerning the ability of the inappropriate pathogens to breach the plant cell wall and infiltrate host cells [45, 46].
Figure 7. F-MWCNTs entry via penetration resulted in cell wall rupture and leaking of plant organelles (a) and being able to agglomerate (b).
Download figure:
Standard image High-resolution imageFigure 7 presented a larger cell wall rupture, due to penetration of functionalized-MWCNTs which resulted in the leaking of plant organelles and their accumulation into the subcellular area of plant cells. The ability of functionalized-MWCNTs to penetrate and rupture the plant cell wall implies their cytotoxicity effect when applied in agriculture. However, functionalized-MWCNTs regardless of size and geometries were observed to accumulate around leaked plant organelles without entering inside. They preferred to be agglomerated with each other, resulting in the cell death.
The direct penetration of functionalized-MWCNTs into plants by their ability to breach the plant cell wall and infiltrate host cells has discarded the cell wall roles to provide tensile strength and protection for plant cell against mechanical stress [47]. Both the plant cell wall and cell membrane should function to control substance movement into and out of the cells. Hence, the plant cells will become selectively penetrable to any foreign ions and molecules. However, different plant [48] and functionalized-MWCNTs combinations have different penetration efficiencies.
Direct penetration of MWCNTs into the seed coat by creating new pores was reported by Khodakovskaya et al [24]. The penetration was claimed to support water uptake inside the seeds and to enhance the growth of tomato seedlings, due to a higher moisture level detected in seeds exposed to MWCNTs compared to unexposed seeds. In agreement Serag et al [35] reported in their work, a marker for endocytosis to label the plasma membrane, that insignificant amounts of plant endosomes showed an association with MWCNTs labelled with the marker. Tripathi et al [49] addressing MWCNTs penetration claimed that functionalized-MWCNTs could be incorporated within the xylem, acting with them, to form several new capillaries which encourage the water uptake potential of the plant in addition to the natural flow. These revealed that entry of MWCNTs into the plant was poorly associated with the endocytosis route, but consistent with direct penetration.
3.5. Chemical analysis of functionalized-MWCNTs, UF-MWCNTs via FT-IR analysis
Figure 8 shows the FT-IR spectra of functionalized-MWCNTs, UF-MWCNTs, and MWCNTs. The spectra mainly attributed to the vibration of the carboxylic acid groups are indicated by bands ∼3000 (O–H stretching vibration), 1700–1725 cm−1 (C=O stretching vibration) and 1210–1320 cm−1 (C–O stretching vibration) [50] for functionalized-MWCNTs as compared to MWCNTs. The present of these new peaks is accredited to the chemical interaction occurring as the result of the HNO3 treatment on the MWCNTs surfaces that produced active acidic functional groups for grafting process with nitrogen from UF.
Figure 8. FT-IR spectra of functionalized-MWCNTs and UF-MWCNTs confirm functional group attachment compared to MWCNTs.
Download figure:
Standard image High-resolution imageThe IR spectra of UF-MWCNTs show significant changes in the peak position and shape of the carboxylic acid. The transmission peak of the O–H stretching vibration ∼3000 cm−1 in the IR spectrum of functionalized-MWCNTs disappeared in the IR spectrum of UF-MWCNTs, indicating that the O–H functional groups might be replaced with the amino groups in the UF during the functionalization process. Also, the sharp peaks of C=O (1739 cm−1) and C–O (1219 cm−1) stretching vibrations of functionalized-MWCNTs were replaced with a new shape of transmission peak that appears to correspond to N–C–N bending ∼1200 cm−1. The bands around 1640–1690 cm−1, 1376–1388 cm−1 and 1120–1290 cm−1 could be assigned to C=O stretching vibration, CH2 bending and C–N stretching. These characteristic spectra are attributed to amide [50]. The chemical interaction between NH2 groups from UF with the carboxyl groups (COOH) on the surface of functionalized-MWCNTs to produce amide groups was also reported elsewhere [51].
The outer walls of MWCNTs are chemically inactive. The functionalization process then activates the sidewalls through the creation of various carboxyl groups on the MWCNTs surfaces. It can be performed at the end caps of nanotubes or at their sidewalls which may have many defects. Hence, the reactions between UF and functionalized-MWCNTs are strongly believed to the formation of covalent bonding between the NH2 groups from UF and carboxyl groups of functionalized-MWCNTs. Thus, the results from the FT-IR spectra confirm that chemical functionalization has occurred between functionalized-MWCNTs and UF instead of physical functionalization (non-covalent) only.
4. Conclusion
The UF-MWCNTs developed via optimum design factors from RSM with CCD show great performances of NUE and NU, hence delivering a promising improvement in efficient rice plant nutrition. The FT-IR spectrum confirms the chemical interaction occurring between the UF and functionalized-MWCNTs through the formation of covalent bonding of NH2 groups on UF-MWCNTs surfaces replacing the carboxyl group. Localization of functionalized-MWCNTs in rice root cells evaluated by TEM imaging investigation shows clear evidence of functionalized-MWCNTs physical translocation through the plant cell phospholipid bilayers. This leads to confirmation of functionalized-MWCNTs uptake via mass flow route with their preference localization at the extracellular region of plant cells. The functionalized-MWCNTs ability to agglomerate again and penetrate the plant cell wall results in cell wall rupture, plant organelle leaking and cell death, alarming their cytotoxicity potential when being used in nanoagriculture.
Acknowledgments
The authors acknowledge Long Term Research Grant Scheme, Ministry of Education Malaysia awarded to Universiti Teknikal Malaysia Melaka Project 3 Carbon Nanotubes-Urea Fertilizer on the Growth of Paddy under the OneBaja Project (Universiti Teknologi Petronas) for supporting and funding this project and the Advanced Manufacturing Centre of Universiti Teknikal Malaysia Melaka for the laboratory and facilities used to complete the studies.