Abstract
In this paper, we present the experimental result as well as the theoretical calculation of the electronic band structures and the optical absorption spectra for N-doped and Fe-doped TiO2 anatase. The main purpose is to provide evidence in the viewpoint of visible light photocatalytic activity of N-doped and weak ferromagnetism of Fe-doped in TiO2 anatase. Accordingly, to evaluate the separate contributions of nitrogen doping and iron doping in anatase, we present the results of spin-polarized density functional theory (DFT) calculations that have been used to calculate the electronic band structures and optical absorption spectra that arise for a range of concentrations of (i) substitutional nitrogen and (ii) substitutional iron in anatase TiO2. Our results show that absorption in the visible range is mainly due to nitrogen states located above the valence bands, whereas weak ferromagnetism of Fe-doped in TiO2 anatase is mainly caused by spin polarization. These results have important implications for the understanding and further development of photocatalytic materials that are active under visible light. These findings agree favorably with our own experimental data and enable conclusions to be drawn about the nature of the practical catalyst in N-doped and the ferromagnetic origin in Fe-doped TiO2 anatase.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Titanium dioxide, TiO 2, has been considered as a promising material for use in dynamic random access memories, dye-sensitized solar cells, photocatalysts for environmental remediation and water splitting, coating materials to obtain superhydrophilic surfaces, and optical devices [1–5]. However, because of the wide band gap of titanium dioxide, its practical application is inhibited by its low photon utilization efficiency and need for an ultraviolet excitation source, which accounts for only a small fraction of the solar lights (about 5%). Therefore, it is an important and challenging issue to develop a new TiO 2 photocatalytic system with enhanced activities under both UV and visible light irradiation compared with bare TiO 2, improving the utilization efficiency of solar energy.
Impurity doping is one of the typical approaches to extending the spectral response of titanium dioxide to the visible light region. Many efforts have been made by modification of titanium dioxide with nonmetals, such as B, C, N, S and F, so as to efficiently extend the photoresponse from the UV to the visible light region [6–11]. Furthermore, some theoretical calculations have been performed to suggest that anion doping of TiO 2 has considerable effects on band gap alteration [8–12]. The success of nitrogen doping and increasing the photocatalytic activity of TiO 2 in the visible light region provides good opportunities for extensive applications, such as oxidation of CO, ethanol, gaseous 2-propanol, acetaldehyde, NO x and the decomposition of dyes, such as methylene blue. It is well known that nitrogen doping can improve the photocatalytic activity of titania in the visible region, while UV photocatalytic activities are seldom studied.
Recently, some metal elements, such as Fe, Cr, Co, Mo and V, have been employed to tune the electronic structure and enhance the photocatalytic activity of titanium dioxide [13–17]. It has been hypothesized that the advantages of the doping of the metal ion in TiO 2 are the temporary trapping of the photo generated charge carriers by the dopants and the inhibition of their recombination during migration from inside the material to the surface, or the enhanced adsorption of the goal pollutants onto the doping ion surface sites. Choi et al [18] reported that the absorption edge of metal-doped TiO 2 shifted to the visible light region. Anpo and co-workers [19, 20] showed that ion implantation of V, Cr and several transition metals shifted the absorption edge to the visible light region. Furthermore, Fe-doped TiO 2 has generated increasing interest as a diluted magnetic semiconductor (DMS) because of its ferromagnetic behaviour well above room temperature for low Fe doping concentrations [21].
In this paper, we studied the electronic structure of N- and Fe-doped TiO 2 nanoparticles by DFT, and characterized them with UV–Vis diffuse reflectance spectroscopy (UV–Vis DRS) and x-ray diffraction (XRD). We believe that knowledge of the electronic band structure is important in understanding the origin of visible light photocatalytic activity of N-doped and weak ferromagnetism of Fe-doped in TiO 2 anatase.
2. Experimental
Fe-doped TiO 2 nano-powders, with iron contents of 1, 3, 6, 10 and 13 wt%, respectively, were prepared by the sol–gel method [22]. Thus, TiO 2 sol was prepared by the controlled sol–gel method with titanium isopropoxide Ti(OCH(CH 3)2)4 (99.0%, Aldrich Co). 3 ml of Ti(OCH(CH 3)2)4 solution in absolute ethanol was added dropwise under vigorous stirring to 46 ml of distilled water. Fe(NO 3)3·6H 2 O was dissolved in distilled water. The TiO 2 sol was slowly added drop-by-drop to an aqueous solution of Fe(NO 3)3·6H 2 O in a water bath with continuous stirring, and the mixture was stirred magnetically for 1 h until it became a reddish clear solution. These samples were calcined at 400 °C for 2 h. For the purpose of comparison, Ti(OH)4 was prepared by the hydrolytic synthesis method. Nitrogen-doped TiO 2 was prepared by heating Ti(OH)4 with urea. One gram of Ti(OH)4 powder was mixed with various amounts of urea granules, heated in a covered crucible in an electric furnace, and then naturally cooled in the furnace. The temperature rate to 400 °C was 25 °C min −1
The structure of Fe- or N-doped TiO 2 samples were determined by x-ray diffractometer D5005 (Siemen) with CuK α radiation. Their morphology and size were investigated with FE-SEM (JEOL). Optical absorption spectra were measured by a V-670 spectrophotometer (Jasco) at room temperature. We also used density fuctional theory (DFT) to calculate the electronic band structures and optical absorption spectra that arise for a range of concentrations of substitutional nitrogen and substitutional iron in anatase TiO 2.
3. Results and discussion
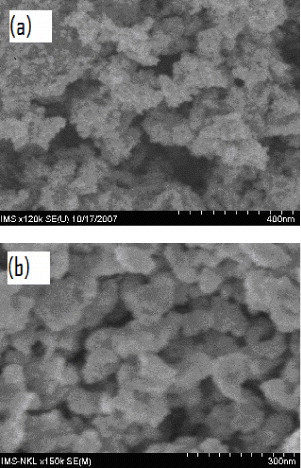
Figure 1 shows SEM images of a typical Fe-doped TiO 2 and TiO 2−x N x . Fe-doped TiO 2 nanoparticles prepared by the sol–gel method have a particle size of about 20 nm. The particle size of TiO 2−x N x samples prepared by the hydrolytic synthesis method was almost the same as that of the Fe-doped TiO 2 samples.
Figure 1 SEM images of Fe-doped TiO 2 (a) and TiO 2−x N x (b).
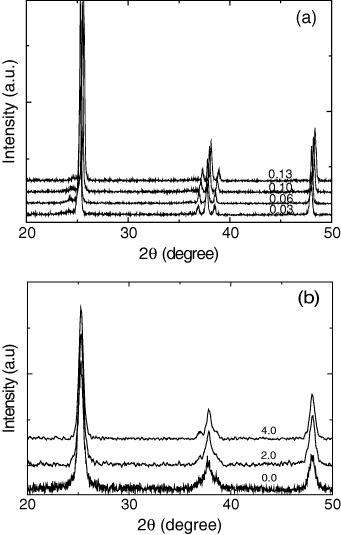
In order to examine the crystallization behaviors of anatase in various samples prepared by the sol–gel and hydrolytic synthesis methods, the XRD 2θ-scan patterns of the samples were measured, as shown in figure 2. XRD reflections of all of the samples exhibited a single phase of anatase. It is noteworthy that no iron oxide or Fe x TiO y peak could be observed in the XRD spectra (figure 2(a)). We think that all of the iron ions were incorporated into the structures of titania and replaced the titanium ion or located at the interstitial site.
Figure 2 X-ray diffraction patterns of Fe-doped TiO 2 with iron contents 1, 3, 6, 10 and 13 wt% (a) and TiO 2−x N x with the weight ratios of urea/Ti(OH)4 of 0, 2 and 4 (b).
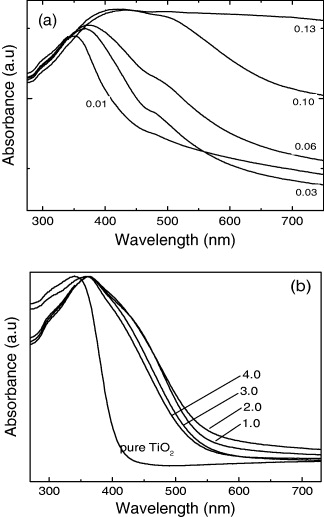
UV–V is diffuse reflectance spectra of TiO 2−x N x and Fe-doped TiO 2 with different Fe doping concentration are presented in figure 3. It is apparent that the diffuse reflectance spectra of all of the samples doped N or Fe have extended a red shift and increased absorbance in the visible range. With increasing Fe-doping concentration, the absorbance increases. Fe-doped TiO 2 showed a shoulder peak at a wavelength of around 590–470 nm, corresponding to absorption energy of 2.1–2.6 eV. However, in the N-doped TiO 2, the absorption energy of the prepared TiO 2 samples was almost the same in spite of changing the weight ratio of urea to Ti(OH)4 from 1 to 4, and the band gap of all samples was about 2.5 eV, corresponding to an absorption wavelength of 500 nm.
Figure 3 The UV-diffuse reflectance spectra of Fe-doped TiO 2 with iron contents 1, 3, 6, 10 and 13 wt% (a) and TiO 2−x N x with the weight ratios of urea/Ti(OH)4 of 0, 1, 2, 3 and 4 (b).
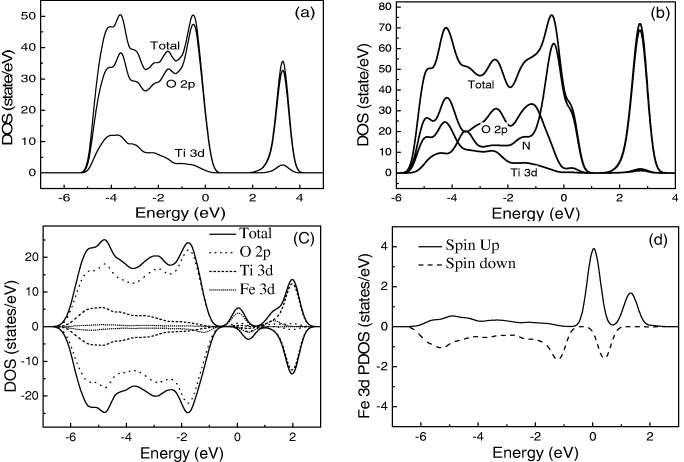
We performed band calculations for anatase TiO 2, TiO 2−x N x (x=0.031 25) and Ti 1−x Fe x O 2 (x=0.0625). Figure 4 shows the density of states (DOS) obtained from the LSDA band calculation. The partial densities of state (DOS) of pure and N-doped anatase are plotted in figures 4(a) and (b). The calculated band gap energy is about 1.96 eV,s which is consistent with that of the bulk anatase TiO 2 crystal model (figure 4(a)). These values are much smaller than the experimentally observed value (3.2 eV) due to the shortcomings of the LDA approach [23]. The projected DOS of the N dopant dominates at the VBM and causes reduction of the band gap of TiO 2 from 1.96 to 1.88 eV for TiO 2 doped 3.125% (figure 4(b)). It is worth noting that with the increase in the nitrogen doping concentration, the contribution of N 2p states increases for impurity energy levels and the part overlapping with the VBM enlarges. However, analysis of the electronic energy levels shows that N-doping causes no shift in the position of both the top and bottom of the O 2p valence band and of the conduction band relative to pure undoped anatase TiO 2.
Figure 4 Density of the states (DOS) of the pure TiO 2 (a), N-doped TiO 2 3.125% (b), Fe-doped TiO 2 6.25% (c) and spin state of Fe 3d in Fe-doped TiO 2 6.25% (d).
Figure 4(c) shows the density of the states of Ti 1−x Fe x O 2 (x=0.0625). The most significant feature in the band structure of Fe-doped TiO 2 was the formation of a new band near the Fermi level. These localized bands formed in the original band gap of TiO 2 came from the Fe 3d orbitals. In Fe-doped TiO 2, the unoccupied Fe 3d orbital also contributed to the conduction band, which was composed mainly of unoccupied Ti 4d, while the occupied Fe 3d orbital contributed to the lower-energy part of the valence band, composed mainly of the O 2p orbital.
It is clear that the pure TiO 2 has an almost symmetrical distribution with spin, whereas the 3d state of Fe in the range from −6 to 2 eV causes an asymmetry of DOS with a spin around the Fermi level. Therefore, every doped Fe atom receives a magnetic moment, hence, we have weak ferromagnetization in Fe-doped TiO 2.
4. Conclusions
We prepared Fe-doped TiO 2 by the sol–gel method and TiO 2−x N x by the hydrolytic synthesis method. Our results show that all of the samples exhibited a single phase of anatase, with the same size of about 20 nm. The results for the calculated electronic structure and experimental optical properties are correlated schematically to describe the possible mechanism of the photocatalytic behavior of the system under study. By spin-polarized density functional theory, we have also found a weak ferromagnetism of Fe-doped in TiO 2 anatase.
Acknowledgment
This work was supported by the Key Project of Ministry of Science and Technology of Vietnam and Hanoi National University of Education.