Abstract
The control of the aging conditions for the aluminium alloys has been considered as an effective strategy to improve the properties of the alloy, especially in enhancement of the corrosion resistance. In this study, a detailed investigation of the effect of one-stage (T6) and two-stage (T76x) aging temper on mechanical properties, exfoliation and intergranular corrosion behaviours of 7075 Al–Zn–Mg–Cu alloy was implemented. Microstructures of the aged alloys were observed by optical microscopy and scanning electron microscopy (SEM), exfoliation and intergranular corrosion behaviours were investigated according to ASTM G34-2001 and ASTM G110-1992, respectively; the mechanical properties of the aged alloys were determined followed the standard methods. The microstructures of the sample aged with the T6 temper showed the finer particle size of precipitates than that of obtained from T76x tempers. Additionally, while the T6-aged sample revealed no appearance of the Precipitate Free Zones (PFZ), the PFZ with 40–50 nm in width was observed in the alloys treated with T76x tempers. The mechanical properties of the sample aged with T76x temper were negligibly reduced comparing to that of obtained with T6 temper, however, the exfoliation and intergranular corrosion behaviours were significantly improved by using two-stage aging conditions. The mechanism in enhancement of the corrosion resistance of the alloys treated with T76x temper over T6 temper was also discussed.
Export citation and abstract BibTeX RIS
1. Introduction
The 2xxx and 7xxx series of aluminium alloys with high strength and duration have been extensively utilized in aerospace industry. In which, 7xxx series (Al-Zn-Mg-Cu) with lower density, higher specific strength, higher resistance to fatigue, are favourable in this application [1–3]. Of these series, aluminium AA 7075 has been proven as one of the most suitable candidates for the aerospace application [4–6]. However, this alloy is prone to the exfoliation and intergranular corrosion, which limited the application of aluminium AA 7075 in some aspects. Thus, it is necessary to improve the corrosion resistance of the alloy to broaden its application.
It is commonly known that the corrosion resistance can be improved by heat treating processes, which consist of one-stage (T6), two-stage (T76), retrogression and re-aging (RRA), however, when the corrosion resistance increase the mechanical properties of the alloys treated with these tempers will be reduced [7–13]. For example, the T6 (one-stage) treatment of 7075 Al alloy could render the resultant alloys with high strength, however the exfoliation and intergranular corrosion resistance are poor. On the other hand, T73, T76 and T74 regimes were developed to improve the localized corrosion resistance, however, they had drawback of decrease in the strength of the alloys.
In order to improve the resistance of localized corrosion as well as remain the high strength of the 7075 alloys, Li et al proposed two novel aging treatments including T6I6 (130 °C, 80 min + 65 °C, 240h + 130 °C, 18h) and high temperature pre-precipitation (HTPP) aging (445 °C, 30 min + 120 °C, 24 h), which showed high tensile strength and improved localized corrosion resistance [14]. The HTPP aging treatment were also utilized by Chen and Huang groups, which revealed the similar outcome [15, 16]. In another study, a treatment process called step-quench and aging, which samples were first quenched to 200 °C–220 °C and kept for 10–30 s before cooled to room temperature (25 °C) and eventually the sample was treated with T6 temper, was proposed by Ou's group [17]. Using this temper, while the mechanical properties were maintained to that of the alloys obtained from T6 temper, the localized corrosion resistance was significantly increased. However, this treatment was not suitable for the Al-7075 alloy. Furthermore, the previous works have not extensively studied the effect of the different keeping time of second stage in the two aging processes T7x on the corrosion behaviours of the Al-7075 alloy. Especially, the cutting-edge technique of digital imaging has not been also employed to investigate the corrosion behaviours yet.
Thus, in order to obtain good localized corrosion resistance such as exfoliation and intergranular corrosion as well as good mechanical properties of 7075 Al–Zn–Mg–Cu alloy, the optimizing conditions for heat treatment are critical. In this work, the effect of keeping time of the second stage in two-stage aging T76x on the properties of the 7075 Al alloys is investigated in comparison to the one-stage aging T6. The mechanical properties, exfoliation and intergranular corrosion behaviours are studied with these various aging tempers. Microstructures of the alloys are thoroughly observed. Importantly, the digital microscope is utilized to have better view on the localized corrosion behaviours of the alloy samples.
2. Experimental section
Al–Zn–Mg–Cu alloys were fabricated with the formula in corresponding to the 7075 aluminium alloy with the chemical compositions as shown in table 1. The obtained alloy bars were extruded at the temperature of approximately 390 °C with feeding rate of 1.6 mm s−1 to obtain the samples with the size of 50 × 50 × 4 mm. The extruded alloy bars were then incubated at 415 °C for 150 min with the rising temperate rate of 5 °C min−1. The alloy bars were naturally cooled down to room temperature (25 °C) before carrying out a temper process at 470 °C for 120 min. The alloy bars after temper were quickly cooled down by immersing in water. The resultant alloy bars were investigated for various aging processes.
Table 1. Chemical compositions of Al–Zn–Mg–Cu (wt%).
| Elements | Fe | Si | Mn | Ni | Cr | Ti | Al | Cu | Mg | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 0.338 | 0.094 | 0.390 | 0.006 | 0.181 | 0.023 | 89.286 | 1.836 | 2.103 | 5.668 |
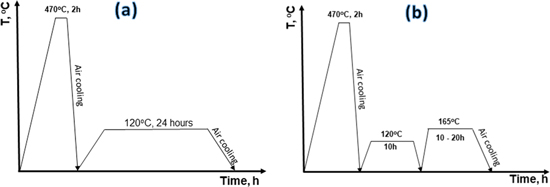
The samples were then treated one-stage and two-stage aging processes as shown in figure 1. The one-stage aging process was adapted from conventional T6 aging treatments (figure 1(a)), which was heated with rising temperature speed of 5 °C min−1 to 120 °C and kept at this temperature for 24 h, then the aged samples were quenched in water [18]. The two-stage aging process as shown in figure 1(b) were carried out as followed: in the first stage, samples were treated at 120 °C for 10 h and cooled down to room temperature (25 °C) before implementing the second stage, which were treated at 165 °C for various keeping time of 10 h (T761), 15 h (T762), and 20 h (T763).
Figure 1. (a) One-stage aging process and (b) two-stage aging process for the Al-Zn-Mg-Cu alloy.
Download figure:
Standard image High-resolution image2.1. Mechanical properties
Hardness of the aged alloys was evaluated by using Rockwell B scale with steel ball diameter of 1.588 mm and weight of 100 kg. The tensile strength of the Al–Zn–Mg–Cu alloy was determined at room temperature (25 °C) using samples with 25 mm in length and a nominal tension rate of 1 mm min−1. The tear experiment was carried out with tension rate of 1.3 mm min−1 using samples oriented in the longitudinal-transverse (L-T) direction [16]. Elongation at break properties were evaluated using ISO 6892-1:2009 approach with sample sizes as shown in table 2. Mechanical properties were repeated for three samples and determined for the average value.
Table 2. Sample sizes for elongation at break testing (ISO 6892-1:2009).
| Properties | Size, mm |
|---|---|
| Specimens length | 35 |
| Width | 10 |
| Thickness | 4 |
| Round Diameter | 7,5 |
| Total length | 118–123 |
| Length of cross section | 50 |
| Length of handle | 25–30 |
| Width of handle | 25 |
2.2. Corrosion investigation
Exfoliation corrosion behaviour was investigated according to ASTM G34-2001 [19]. Typically, samples with the size of 50 × 100 mm were polished with sandpaper from coarse to fine sand particles, then sample was risen thoroughly with distilled water, dried and weighted to determine the initial weight of sample. The compositions of corrosive solution consisted of NaCl 4M, KNO3 0.5M, HNO3 0.1M and water. The solution pHs was calculated approximately to be 4. The prepared specimens were immersed in the solution for 48 h at the temperature of 25 ± 3 °C. After corrosion process, the specimens were taken out, washed thoroughly with water, dried and determined the weight. The specimens were kept in vacuum for further characterizations.
Intergranular corrosion behaviour was evaluated according to ASTM G110-1992 [20]. Specimens with the size of 8 × 6 × 4 mm were polished with sandpaper from coarse to fine sand particles, then specimens were washed thoroughly with distilled water and dried in vacuum. 1 liter of corrosion solution was prepare by dissolving 57g NaCl with 10 ml H2O2 (30%) and water. The specimens were immersed in the prepared corrosion solution for 6 h at temperature of 30 ± 3 °C. After reaction, the etched specimens were taken out, risen thoroughly with distilled water and polished with fine sandpaper. The depth of corroded alloys was observed by electron scanning microscopy with magnification of 500 times.
2.3. Micorstrcuture observation
Microstructures of the aged alloys were first observed by optical microscopy (Axiovert 25 CA, Carl Zeiss - Germany), scanning electron microscopy (SEM - EDX Hitachi S-4600), and high resolution transmission electron microscopy (TEM, FEI Tecnai, USA). The crystallinity of the treated alloys was investigated by x-ray diffraction (XRD, Smartlab Rigaku, Japan). The cutting-edge technology of digital microscopy (VHX-6000, Keyence, Japan) was also employed to further observe microstructures with colour bar, that describe to seriousness of damage caused by intergranular corrosion. Precipitates in the matrix as well as along grain boundary were observed by SEM.
3. Results and discussion
3.1. Microstructures
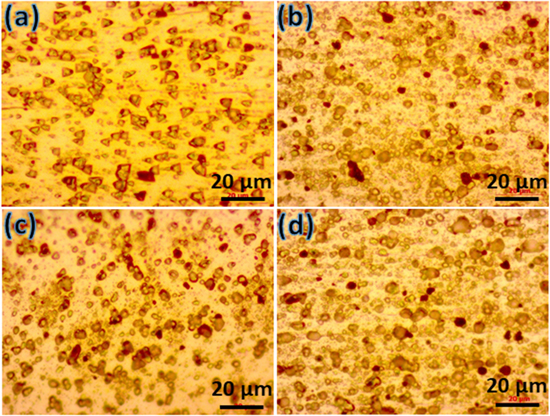
The microstructures of Al-Zn-Mg-Cu alloy prepared from various aging conditions were firstly studied by optical microscope as shown in figure 2. In the traditional T6 aging process (figure 2(a)), many uniform triangle-shaped phases are observed on the surface of the aged alloy, several spherical phases are also presented. When aging with 2-stage process (figures 2(b)–(c)), it can be clearly seen that the number of phases with spherical shape are higher than the presence of the triangle shape on the surface of the Al alloys. Prolonging in the second stage of 2-stage aging (from 10 to 20 h) witnesses the increase of the precipitates size, which is larger than that of obtained from T6 aging process. This can be explained that the 2-stage aging process is of the second stage at high temperature of 165 °C, thus uniform and small phases formed at the first aging stage at low temperature of 120 °C for 10 h continue to develop to more stable state with no existence of intermediate granular phases, even aggregates to minimize free energy if the retaining time of second stage is long enough. When increasing the aging time at second stage of 2-stage process, while more intergranular phases and bright region are observed, the fine phases and density of phases are significantly decreased.
Figure 2. Optical micrographs of specimens prepared from various aging conditions: (a) T6, (b) T761, (c) T762, and (d) T763.
Download figure:
Standard image High-resolution imageIn order to observe the boundary phases generated from α solid state and discontinuous phase on the boundary of the particles, which were not able to observed by optical microscope, the scanning electron microscopy (SEM) images with magnification of 50,000 times and transmission electron microscopy (TEM) images were obtained as shown in figures 3 and 4. It is obvious from the figures that with the alloy obtained from the T6 temper, the high density of fine phases is homogeneously distributed and precipitated in the matrix with the size ranging from 5 to 20 nm (figures 3(a) and 4(a)). On the grain boundary, the majority of precipitates are η phases with the size of larger than in the matrix, which are continuously distributed. The Precipitate Free Zones (PFZ) is not observed in the alloy surface aged with the T6 temper (figures 3(b) and 4(b)). When the alloy was treated with the T762 temper, the low density of phase η with larger size in comparison with that obtained from T6 temper is observed as shown in figures 3(c) and 4(c). The precipitates of phase η on the surface of alloy treated with T762 temper are coarse and discontinuously distributed with average size of around 100 nm. Interestingly, heat treatment with T762 temper witnesses the formation of PFZ zones with 40–50 nm in width, which can be clearly seen in the figure 3(d). This is because in the second stage aging of T762 heat treating process, the high temperature (165 °C) enables the rapid development of stable phase η, which appeared in the first stage of aging, located continuously on the grain boundary. These phases η after two-step aging become coarse and discontinuous to minimize the free energy. Especially, the area around grain boundary, where has many elements such as Mg, Zn and diffusion processing on the grain boundary is easier than in the matrix (figure 4(d)). Therefore, stable phases η developed very easily and became coarse leading to the discontinuous distribution at the grain boundary.
Figure 3. SEM images with magnification of 50,000 times of specimens prepared from various aging conditions: (a) and (b) T6, (c) and (d) T762.
Download figure:
Standard image High-resolution imageFigure 4. TEM images of 7075 Al alloys prepared from various aging conditions: (a) and (b) T6, (c) and (d) T762.
Download figure:
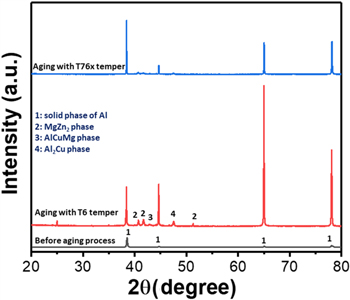
Standard image High-resolution imageCrystallinity of the prepared 7075 Al alloys were investigated by using x-ray diffraction (XRD). Figure 5 shows the XRD patterns of the alloy samples before and after aging processes with T6 and T76x tempers. It is obvious from the XRD pattern of Al alloy before aging that only diffraction peaks of the α phase of Al could be observed, the peak intensities of the alloyed phases of MgZn2 and Al2CuMg are negligible. However, after aging with the T6 temper, beside the appearance of characteristic peaks of α phase from Al with significantly higher intensities than that of Al alloy sample before aging, the diffraction peaks of η, s, and θ phases are belonged to the MgZn2, Al2CuMg, and Al2Cu crystals, respectively [21]. In which, η is the principal phase to increase the mechanical properties of the 7075 alloy, however, S phases (Al2CuMg and Al2Cu crystals) are also durable phases which enhance mechanical properties of the 7075 alloy [22]. Importantly, the presence of the S phases significantly improves the corrosion resistance of the 7075 alloy as different surface potential of the alloy decreases [23]. These phases are also observed in the XRD patterns of the Al alloy after treated with T76x temper, however the intensities of MgZn2, Al2CuMg, and Al2Cu phases are relatively lower than those phases of the alloy obtained from the sample treated with the T6 temper. This might be ascribed to the short aging time at the first stage (120 °C, 10 h) of two-stage T76x temper in comparison to that of the one stage T6 temper (120 °C, 24 h), which leads to the lower crystallinities of the Al, MgZn2, Al2CuMg, and Al2Cu phases in 7075 Al alloy treated with T76x temper.
Figure 5. XRD patterns of the 7075 Al alloys before aging process (grey line) and after treated with T6 (red line) and T76x (blue line) tempers.
Download figure:
Standard image High-resolution image3.2. Mechanical properties
The mechanical properties of the alloy treated with one-stage and two-stage aging processes are shown in figure 6. It is obvious that the mechanical properties of alloys treated with the T76x temper is decreased. Particularly, the tensile strength of the sample used T76x temper decrease around 13% in comparison with that of treated with T6 temper, which decreases from 585.3 MPa (T6) to 526 MPa (T761) (figure 6(a)). Further increase in aging time from 10 to 20 h at second stage of T76x only causes a slightly decrease in the tensile strength. This trend is also observed with the elongation and harness of the alloy treated with various heating procedures (figures 6(b) and (c)). The elongation and the hardness of the samples treated with the T76x temper decrease approximately 13 and 10%, respectively, in comparison with sample treated with the T6 temper. This is probably ascribed to short aging time (10h, 120 °C) of the first stage in T76x temper in comparison to that of the T6 temper (24h, 120 °C), which make the decrease of elongation. In the T76x temper, the aging time also has negligible impact on the elongation and the hardness of the alloys. These decreased mechanical properties of the alloys treated with T76x temper are ascribed to the heavily over-aged microstructure. While two-stage aging leads to decrease the mechanical properties, the exfoliation corrosion resistance of the alloys is significantly increase. It can be clearly seen from the figure 6(d) that the weight losses of the samples treated with the T76x temper decrease around 20% compared to the samples treated with the T6 temper, indicating that the exfoliation corrosion resistance of the alloy is significantly enhanced with the two-stage heat treating process.
Figure 6. (a) Tensile strength (MPa), (b) elongation (%), (c) hardness (HRB), (d) weight losses of the samples immersed in corrosive solution (NaCl 4M, KNO3 0.5M, and HNO3 0.1M) with various aging conditions.
Download figure:
Standard image High-resolution image3.3. Exfoliation corrosion
While two-stage aging leads to decrease the mechanical properties, the exfoliation corrosion resistance of the alloys is significantly increased. It can be clearly seen from the figure 6(d) that the weight losses of the samples treated with the T76x temper decrease around 20% compared to the samples treated with the T6 temper, indicating that the exfoliation corrosion resistance of the alloy is significantly enhanced with the two-stage heat treating process.
The visual view of the alloys surface obtained by various aging conditions after exfoliation corrosion test is shown in figure 7. It is obvious in all samples that when the samples were immersed in the exfoliation corrosion testing solution, the foams and gasses on the surface of the alloys were formed immediately indicating the samples are corroded quickly. The rough and heavy damage of the alloy surface obtained by the T6 temper indicates the serious exfoliation corrosion occurred, the fine particles are peeling off the surface of the sample and settle to the bottom of the solution and appear many black streaks and holes on the surface of the specimen (figure 7(b)). When heat treating with the T76x temper, the exfoliation corrosion of the alloys was also occurred, however this phenomenon is much slower than the sample treated with the T6 temper as can be seen in figures 7(c)–(e). It can be also seen that in the T76x heat treatment, the heating time at second stage significantly increases the exfoliation corrosions resistance of the alloys. The optimized heating time can be seen to be 15 h at 165 °C as finer surface is observed (figure 7(d)). These results are consistent with the determined weight losses mentioned above. This phenomenon can be explained that for the T6 treatment, many metastable fine phases η' (MgZn2) occur within grain with high density and be rich in element Mg, so these Mg-rich intermetallics are anodic with respect to the matrix and preferentially dissolve in corrosive solution, as a result, the alloy treated with T6 temper is corroded quickly. For the over-aging treatment with T76 temper, phase η is more coarse, lower precipitation density than the T6 treatment, thus the alloys treated with the T76x tempers corrode slowly. With the T76x treatment, the increase of aging time from 10–20 h leads to formation of coaser MgZn2 particles, as a result, less corrosive behaviour is observed on the surface. After 15 h of aging time, the size of MgZn2 particles is quite stable leading to further increase of aging time has negligible effect on the exfoliation corrosion resistance of the alloy. The visual observation was employed to determine the exfoliation corrosion (EXCO) rating of P (pitting corrosion), which is devided into PA, PB, and PC (very severe pitting corrosion) [24]. The EXCO rating of the sample treated with T6 temper can be assessed to be PC as servere corrosion pitting was observed. The EXCO resistance is significantly improved for the samples treated with T76x tempers. While the Al alloy treated with the T761 temper exhibits the estimated EXCO rating of PB with the reasonable corrosion pitting damage (figure 7(c)), the alloys treated the T762 and T763 temper reveal the slight pitting corrosion on the surface, which is assigned to the EXCO rating of PA.
Figure 7. Optical images before (a) and after exfoliation corrosion process for 48 h of the samples treated with various aging processes: (b) T6, (c) T761, (d) T762 and (e) T763.
Download figure:
Standard image High-resolution image3.4. Intergranular corrosion
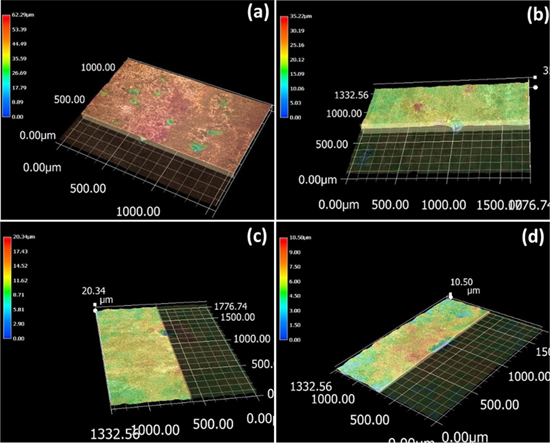
The intergranular corrosion behaviour of the alloys treated with the one-stage T6 and the two-stage T76x tempers was first investigated by observing the cross-section scanning electron microscopy (SEM) images at the corrosion position. The results are shown in figure 8. It can be seen that the sample with the T6 temper is greatly intergranularly corroded with the corrosion depth of approximately 56.4 μm (figure 8(a)). However, when aging with the T76x temper the intergranular corrosion resistance is significantly improved (figures 8(b)–(d)). The corrosion depths are determined to be 19.67, 15.32, and 7.33 μm for the samples treated with the T76x temper with aging time at second stage of 10, 15, and 20 h, respectively, which are reduced by 286, 368, and 769% in comparision with the alloy treated by the T6 temper.
Figure 8. The cross-section SEM images of the 7075 alloy surfaces treated by different conditions of aging (a) T6, (b) T761, (c) T762 and (d) T763 after the intergranular corrosion.
Download figure:
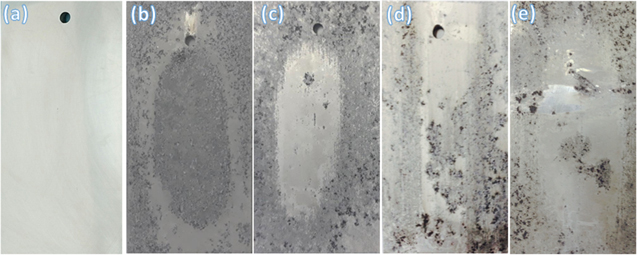
Standard image High-resolution imageThe enhancement in intergranular corrosion resistance by the aging treatment is further confirmed by digital observation as shown in figure 9. It can be clearly seen that the surface of the alloy aged with the T6 temper is seriously damaged by the intergranular corrosion (figure 9(a)) as the whole surface of the alloy is covered with the organe colour. The uniform green colour can be clearly observed on the surface of the alloys aged with the T76x temper indicating the improve resistance in intergranular corrosion of the 7075 aluminium alloys. These results are in well-agreement with the cross-section SEM images, where the depth of intergranular corrosion is significantly reduced with the alloys treated by second-stage aging conditions. This improved corrosion resistance is due to the appearance of the PFZ zones on the surface of alloys treated with T76x tempers as seen in the figures 3 and 4. Further mechanism on the enhanced corrosion resistance is discussed in the following section.
Figure 9. Digital images of the 7075 alloy surfaces treated by different conditions of aging (a) T6, (b) T761, (c) T762 and (d) T763 after the intergranular corrosion.
Download figure:
Standard image High-resolution imageIt is common belief that the continuous grain boundary precipitates (GBP) are harmful to corrosion properties of the alloy [25, 26]. The continuous GBP is preferentially dissolved as anodes in anodic dissolution. The potential of GBP, PFZ and matrix are −1.05 V, −0.85 V and −0.75 V, respectively, which indicates that the potential difference between GBP and PFZ is less than that of between GBP and the matrix. With regard to corrosion resistance of the alloy, widening PFZ can remit the corrosion sensibility and improve corrosion resistance of alloy. With T6 temper, stable phases η is continuous distribution at the grain boundary, there are no Precipitate Free Zones (PFZ) along the two side of the grain boundary, as a result, the alloy treated with the T6 temper was facilely exposed to the corrosive environment, the phase η on the grain boundary preferentially dissolved due to the galvanic corrosion reaction between phase η and adjacent alloy matrix and resulted in an active corrosion path on the grain boundary. With the T76x temper, stable phases η are coarse, discontinuous distribution at the grain boundary. In addition, along the two sides of the grain bourndary appear the PFZ zone, which is wide in case of the high over-aging procedure. Thus, the samples aged with the T76x are slightly corroded at the grain boundary.
4. Conclusions
The effects of one-stage and two-stage aging processes of 7075 aluminium alloy on microstructure, mechanical properties, exfoliation and intergranular corrosion were successfully studied in detail. The results indicate that the main aging precipitates in the aluminum matrix of the T6 temper are η' phase, which is metastable fine phase, high density and η phase is bigger size, lower density. On the grain boundary η precipitates are larger than that within matrix, continuous distribution, no PFZ region appeared along the two sides of the grain boundary. In contrast, the alloys treated with two-stage T76x temper contain many η stable coarse phases with the size in range of from 20 to 30 nm and small number of η' metastable fine phases with size of smaller than 10 nm, which is distributed randomly between η phases. The PFZ region is observed along the two sides of the grain boundary on the surface of the alloys treated with the T76x temper. While, the mechanical properties of the alloys treated by two-stage aging process are reduced insignificantly in comparison to that of obtained one-stage aging, the exfoliation and intergranular corrosion resistance of the alloys treated with T76x temper are remarkably improved. These results will enable capability of manipulating the properties of the 7075 aluminum alloy for the wider range of applications, especially in the aerospace industry.
Acknowledgments
The authors acknowledge the staffs and facilities at Hanoi University of Science and Technology and Institute of Chemistry and Materials for the support in characterizations.