Abstract
In this study we have successfully deposited Ag-SiO2 co-doped TiO2 thin films on glass substrates by the sol–gel method. After being coated by a dip coating method, the film was transparent, smooth and had strong adhesion on the glass surface. The deposited film was characterized by x-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), ultraviolet-visible spectroscopy (UV–Vis), a scanning electron microscope (SEM) and atomic force microscope (AFM) to investigate its crystallization, transmittance and surface structure. The antifogging ability is explained by the contact angle of water on the surface of the glass substrates under visible-light. The obtained results show that Ag-SiO2 co-doped TiO2 film has potential applications for self cleaning and anti-bacterial ceramic tiles.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Glass and ceramic tiles are widely employed in industry. They are mainly used as construction and transportation materials. In most cases, we need a periodical cleaning process to maintain their transparency as well as their appearance. It is costly and dangerous for workers to clean glass or ceramic surfaces on the outside of a building. Therefore there is the need for self-cleaning glass and ceramic tiles. Titanium dioxide (TiO 2) is a nontoxic material and has been applied in environmental treatments such as water and air purification, water disinfection and sterilization because of its unique properties such as strong photocatalytic activity and chemical stability [1]. The mechanism of photocatalytic activity of TiO 2 has been studied extensively, as shown in the literature [2, 3]. The most widely accepted mechanism is the migration of valence electron to conduction band and the formation of hole–electron pairs. These hole–electron pairs react with adsorbed molecules at the semiconductor surface, resulting in the degradation of adsorbates [3]. Titania is able to kill microorganisms; therefore it is used as a biocide [4, 5]. However, major limitations of TiO 2 are the absorption region in the UV and fast recombination of hole–electron pairs within nanoseconds. We can use additives such as Pt, Pd and Au to improve the photocatalytic efficiency of TiO 2 [6]. These additives capture electrons to prevent the fast recombination of hole–electron pairs. Silver is a suitable and nontoxic element which improves the TiO 2 bioactivity because of its inborn antibacterial activity against different microorganisms [7].
The introduction of a second metal oxide (SiO 2, ZrO 2, Al 2 O 3, etc) was found to be an effective route to improve the thermal stability and UV photocatalytic activity of TiO 2 [8]. Among them, SiO 2-TiO 2 and ZrO 2-TiO 2 systems were most widely investigated in the photocatalysis field. They exhibited higher photocatalytic activity than pure TiO 2. This could be explained as follows: the addition of SiO 2 or ZrO 2 in TiO 2 enhanced the thermal stability for the phase transformation of TiO 2 particles from anatase to rutile, and also increased the surface area, pore volume and surface acidity. Additionally, according to previous reports [8, 9] the presence of SiO 2 could increase the adhesion, the mechanical stability of the film on substrates, enhance the superhydrophilicity of the film surface and maintain its superhydrophilicity longer in dark places. In addition, the effects of SiO 2 content on the textural, optical, surface electronic state and photocatalytic activity of the Ag-SiO 2 co-doped TiO2 were also presented.
2. Experiments
The following chemicals were used: Tetra-isopropyl ortho titanate Ti(OC 3 H 7)4 (TIPOT-Merk), Tetra-ethoxyortho silicate Si(OC 2 H 5)4 (TEOS-Merck), EthanolC 2 H 5-OH (Merck), Hydrochloric acid, HNO 3 (China), Silver nitrate AgNO 3 (Merck), distilled water. The synthetic process of the Ag-SiO 2-TiO 2 solution is shown in figure 1.
Figure 1 The synthetic process of the N-SiO 2-TiO 2 solution.
The volume of H2O containing 1 wt% HNO3 can be determined by the formula

The optimum concentration of the molar ratio SiO 2/(SiO 2+TiO 2) is 15% [10]. In this study, we synthesized the Ag-SiO 2-TiO 2 coating solution with various Ag/(SiO 2+TiO 2) molar ratios: 1–10%. Ag-TiO 2/SiO 2 thin films were deposited on substrates by a dip coating process at room temperature. Glass slides with dimensions of 26×76 mm 2 were used as substrates. Before the deposition, the substrates were ultrasonically cleaned in dilute HCl, acetone and absolute ethanol for 30 min, respectively. Finally, they were thoroughly rinsed with distilled water.
The substrates were immersed into as-prepared Ag-TiO 2/SiO 2 sol for one min. The substrates were then withdrawn from the sol with velocity 4000 rpm. If coating two times or more, each layer would be dried between two successive coatings at 200 °C for 5 min before the next coating was implemented. Afterward, the substrates were calcined at 500 °C for 1 h.
X-ray diffraction (XRD) patterns of these powder samples were measured with a diffractometer (D8 Advance). The samples were characterized using SEM observation (Field Emission Scanning Electron Microscope, Jeol JMS-6480LV). An atomic force microscope (AFM-Electronica S.L) was used to investigate the crystallization and surface structure. Synthesized samples were also studied using UV–vis absorption spectra with a Jasco UV–vis V530 double beam spectrophotometer in a wavelength range from 190 to 1100 nm, and Fourier transform infrared spectroscopy (FTIR) Tensor TM 37 (Bruker) in the range 400–4000 cm -1 by the KBr pellet technique.
3. Results and discussion
3.1. UV–VIS spectra
The optical transmittance spectra of Ag-TiO 2/SiO 2 films with different ratios of Ag (1–10% mol) are shown in figure 2. The transmittance within the visible and near infrared region is higher than 85% including glass substrates, which reveals the superior optical properties of Ag-TiO 2/SiO 2 produced in this work. The transmittance slightly decreases with increase of the Ag ratio. In all cases, the films present a sharp absorption edge in the ultra violet region at a wavelength of about 350 nm, and the absorption edge shifted towards longer wavelengths (red shift) with the increase of Ag concentration from 1 to 7 mol%, especially with the result Ag-TiO 2/SiO 2 with Ag 7 mol% thin film absorb wavelength at 417.5 nm (figure 2) in the visible region. A shift towards shorter wavelengths (blue shift) is observed when the amount of Ag-doping is more than 7 mol%. With this result, this material system can be applied to research and manufacturing photo-catalyst TiO 2 nanomaterials in the visible light range.
Figure 2 Transmittance spectra of Ag-TiO 2/SiO 2 film with different ratios of Ag 0–10%.
3.2. X-ray diffraction
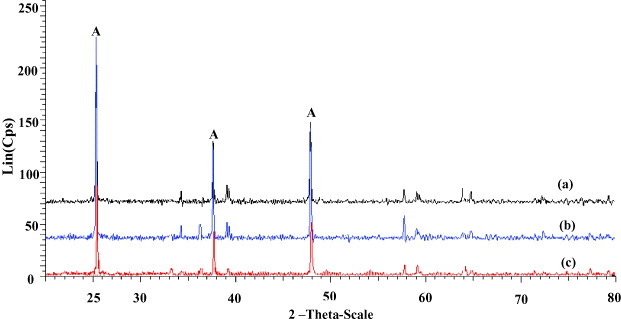
X-ray diffraction patterns of Ag-TiO 2/SiO 2 powder with 0% Ag (a), 7% Ag (b) and 10% Ag (c) are shown in figure 3. The remarkable peaks observed at 2θ=25.3 o , 48.12 o , 53.94 o , 55.04 o and 62.74 o are assigned to (101), (200), (105), (211) and (204) reflections of anatase phase of TiO2, respectively. There is no remarkable diffraction peak of SiO2 because it is amorphous.
Figure 3 XRD pattern of Ag-TiO 2/SiO 2 powders annealed at 500 °C for 1 h.
The particle sizes can be calculated by the Scherrer equation

where k=0.89, λ is the wavelength (λ=0.1541 nm), θ is the half-diffraction angle, β is the half-peak width, and D is the diameter of the crystalline particle. The estimated average crystal size of sample (a), (b) and (c) (calculated directly from figure 3) is assigned to 13, 17 and 25 nm, respectively.
3.3. Infra-red spectroscopy
Figure 4 shows the FTIR spectra of Ag-TiO 2/SiO 2 containing Ag (a) 0%, (b) 7% and (c) 10%. The absorption peak is seen at 3000–3800 cm -1, which is assigned to the stretching vibration of the O-H bond in water molecules absorbed at the surface. The peak at about 1600 cm −1 is attributed to the bending vibration of the H-O-H bond, which is assigned to the chemisorbed water. The peak at approximately 440 cm −1 is due to the stretching vibration of the Ti-O-Ti and Ti-O bonds.
Figure 4 FTIR spectra of Ag-TiO 2/SiO 2 containing 0, 7 and 10% mol Ag heat treated at 500 °C.
Two absorption peaks are seen at about 950 and 1050 cm −1 which are ascribed to the asymmetric stretching vibrations of the Ti-O-Si and Si-O-Si bands, respectively. We have not found the existence of Ag in figure 4. Perhaps Ag is located in the crystalline network. (Intercalate dope.)
3.4. Microstructures
The AFM images in figure 5 indicate that the particle size and film roughness decreased with the increase of Ag. The surface roughness (RMS) decreased from 4.65 nm to 1.57 nm and 1.03 nm with the addition of Ag from 0% to 7% and 10%, respectively. Therefore, the amount of doped Ag prevented the crystallization of TiO 2 powders.
Figure 5 AFM images of 0% (a), 7% (b) and 10% (c) Ag-SiO 2/TiO 2 films.
Figure 6 shows a SEM photograph of the surface of Ag-TiO 2/SiO 2 with Ag 7% thin film calcined at 800 °C for 1 h. At 15 000 magnification count, we couldn't observe the particles size of TiO 2, but we can see that surface Ag-TiO 2/SiO 2 thin film is fairly even and without cracking.
Figure 6 SEM photograph of the surface of Ag-TiO 2/SiO 2 with Ag 7% thin films calcined at 500 °C.
3.5. Super-hydrophilic property and self-cleaning effect
To investigate the antifogging ability of coating films, samples were exposed to hot water vapor. Figure 7 shows the result of this test with visible light exposure. Glass substrate (a) and Ag-TiO 2/SiO 2 films with Ag 0, 7 and 10% are assigned to (b)–(d). With the Ag-TiO 2/SiO 2 film coated glass sample (b)–(d), we can read the letter behind very clearly. However the letters behind the glass substrate sample without coating (a) cannot be read. This results show the excellent antifogging ability of Ag-TiO 2/SiO 2 films produced in this work. These transparent self-cleaning TiO 2 films on glass substrates have great potential for practical applications such as in mirrors, window glass and the windshields of automobiles.
Figure 7 The antifogging ability of Ag-TiO 2/SiO 2 films on glass substrate.
When the surface of TiO 2 film is exposed to visible light, the contact angle of the TiO 2 film with water decreased gradually. After adequate exposure to light, the surface reaches super-hydrophilic (2 o < water contact angle). In this case, electrons and holes are still produced, but they react in a different way. Electrons tend to reduce Ti (IV) cations to the Ti(III) state, and holes oxidize O − 2 anions. In the process, oxygen atoms are ejected, creating oxygen vacancies. Water can then occupy these oxygen vacancies and produce adsorbed OH groups, which tend to make the surface hydrophilic [11].
Figure 8 shows the result for Ag-TiO 2/SiO 2 films with 0 and 7% Ag placed in the dark then measured at different times. We can see that thin film 7% Ag could maintain the super-hydrophilic ability for a long time. In Ag-doped TiO 2/SiO 2 the recombination time of electron–hole pairs is large, which extends photocatalysis.
Figure 8 Graph of the contact angle versus time.
4. Conclusion
Ag-SiO 2 co-doped TiO 2 solutions with high stability have been prepared by a simple sol–gel method and may be utilized for mass production. An optimum concentration of Ag is 7% at 500 °C temperature of substrates. It was found that doped silver increases the photosensitivity of nano TiO 2 with rather high retention time in characteristics. The as-prepared material can be used to manufacture TiO 2 operating in the region of visible light.
Acknowledgments
The authors express their thanks for the support from the Laboratory for Nanotechnology (LNT)-Vietnam National University (VNU), Ho Chi Minh City.